Valence shell of nitrogen
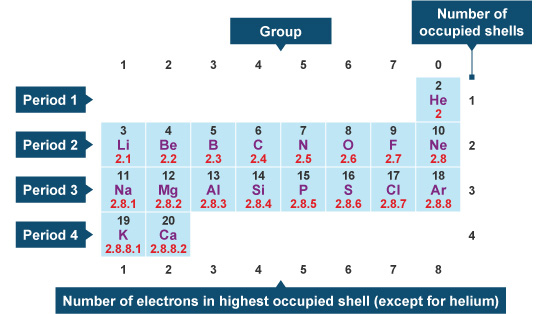
Nitrogen has 5 valence electrons, valence shell of nitrogen. The thing to remember about main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, "N"is located in group 1color red 5which means that it has color red 5 valence electrons.
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. Nitrogen is in Group 5, so it has 5 outer shell electrons. How many valence electrons does nitrogen have? Doc Croc.
Valence shell of nitrogen
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure. Wave Function. Molecular Orbitals. Sigma and Pi Bonds. Bonding Preferences. Formal Charges. Skeletal Structure. Lewis Structure. Condensed Structural Formula. Degrees of Unsaturation. Constitutional Isomers.
Related Videos. Atomic Structure. Diazo Replacement Reactions.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table.
Valence shell of nitrogen
Nitrogen is the 7th element in the periodic table and the first element in group The standard atomic mass of nitrogen is Nitrogen participates in the formation of bonds through valence electrons. This article discusses in detail how to easily calculate the number of valence electrons in nitrogen.
How to make piston doors in minecraft
Phenols 15m. Calculations with Enantiomeric Percentages. You can reuse this answer Creative Commons License. Bonding Preferences. IR Spect:Extra Practice. Addition Reactions 3h 18m. Intermolecular Forces. Eglinton Reaction. How do valence electrons determine chemical reactivity? Acids and Bases 2h 45m. Naming Aldehydes. Stefan V. Moving Functionality. How many valence electrons does oxygen have?
If you're seeing this message, it means we're having trouble loading external resources on our website.
Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. Aldehydes and Ketones:Nucleophilic Addition 4h 52m. Acid Base Equilibrium. Nitrogen has two naturally occurring isotopes, nitrogen and nitrogen, which can be separated with chemical exchanges or thermal diffusion. For dinitrogen to follow the octet rule, it must have a triple bond. The compound is also very inert, since it has a triple bond. Enantiomers vs. Carbocation Intermediate Rearrangements. Mass Spect:Isotopes. This is the case because adding 3 electrons to nitrogen's valence shell will give it a complete octet. How many valence electrons does oxygen have? Sigmatropic Rearrangement. Para Positions. Diazo Sequence Groups.

Rather amusing piece