Velocity of an electron in nth orbit
Angular momentum.
The velocity of the electron in the first Bohr's orbit is 2. The linear velocity of electron in third orbit is. The velocity of electrons in the ground state of H- atom is 2. The velocity of an electron in the first Bohr orbit of hydrogen atom is 2. Its velocity in the second orbit would be.
Velocity of an electron in nth orbit
Derive an expression for the velocity of an electron revolving round the nucleus in the orbit of hydrogen atom. From Bohr postulate, the centripetal force is provided by the electrostatic force between the nucleus and the electron, i. Byju's Answer. Open in App. By assuming Bohr's postulates derive an expression for radius of n t h orbit of electron, revolving round the nucleus of hydrogen atom. Derive an expression for critical velocity of a satellite revolving around the earth in a circular orbit. On the basis of Bohr's postulates derive an expression for orbital velocity of an electron in n th stationary orbit of hydrogen atom. What will be the kinetic energy of the electron in this state? An electron in the ground state of hydrogen atom is revolving in anticlockwise direction in a circular orbit of radius R. Obtain an expression for the orbital magnetic moment of the electron. The frequency of an electron is n when it is revolving around nucleus of hydrogen atom in 1 s t orbit. The frequency of same electron when it is revolving in 2 n d orbit is. Matter Waves. Standard XII Physics.
The velocity of an electron in the first orbit of H-atom is v. If the speed of electron in the first bohr orbit of hydrogen atom is x
For electron moving in n t h orbit of the atom , the angular velocity is proportional to:. If an electron is moving in nth orbit of H-atom, then its velocity is-. In Bohr's orbit , kinetic energy of an electron in the n t h orbit of an atom in terms of angular momentum is propotional to. The magnetic field induction produced at the centre of orbit due to an electron revolving in n t h orbit of hydrogen atom is proportional to. The angular speed of the electron in the n t h Bohr orbit of the hydrogen atom is proportional to.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Atoms and electrons. How Bohr's model of hydrogen explains atomic emission spectra. Key points. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells , or orbits, around the nucleus. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. Bohr's model does not work for systems with more than one electron. The planetary model of the atom.
Velocity of an electron in nth orbit
Hey there! We receieved your request. Various other concepts like radiation less orbits and stationary states were also introduced in the Bohr Model. We discuss the postulates of Bohr Atomic theory. Bohr introduced the concept of radiation less orbits in which the electrons revolve as usual around the nucleus but without radiating any kind of energy which is contrary to the laws of electromagnetism. This was a hypothesis, but at least a working one. Radiation occurred only when an electron made a transition from one stationary state to another.
X2 11x 24
What will be the ratio of de - Broglie wavelengths of proton and alpha Phone Number. Planck's constant. I am a lecturer and a visiting faculty with different universities. Medicine and Allied Sciences Change. Video Answer Solved by verified expert. The circumference of the first Bohr orbit in H atom is 3. Post Answer. English Hindi. An electron jumps from the 3rd orbit to the ground orbit in the hydrog Step-by-step Solved, Expert Educator: The velocity of an electron in the nth orbit of a hydrogen. Sign Up. The following is the relation between the object distance and the image distance in a plane mirror image :. Please add your first playlist.
Bohr's model of the atom was valuable in demonstrating how electrons were capable of absorbing and releasing energy, and how atomic emission spectra were created. However, the model did not really explain why electrons should exist only in fixed circular orbits, rather than being able to exist in a limitless number of orbits with different energies. In order to explain why atomic energy states are quantized, scientists had to rethink their views of the nature of the electron and its movement.
Sign Up Free. The velocity of an electron in the first Bohr orbit of hydrogen atom i Its velocity in the second orbit would be. Create Your Account Name. An electtron and a photon have same wavelength. A The wavelength of the Kalpha line ofthe characteristic X rays emitt Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. The velocity of an electron in the nth orbit of a hydrogen atom is 2. Millions of real past notes, study guides, and exams matched directly to your classes. Suggested Test Series. Byju's Answer. Mobile No. Your personal AI tutor, companion, and study partner. In hydrogen atom, if the difference in the energy of the electron in n

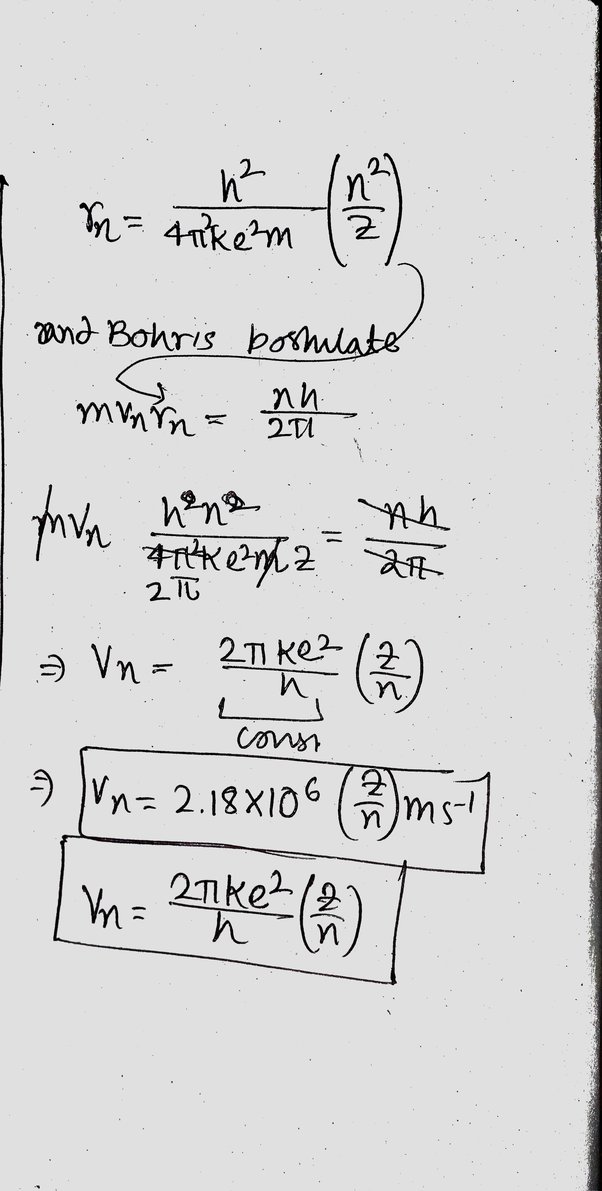
Many thanks for the help in this question, now I will not commit such error.
Certainly, certainly.
Earlier I thought differently, thanks for an explanation.