What is neutralisation reaction give two examples
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait. The chemical opposite of an acid is a base. These original definitions were proposed by Arrhenius the same person who proposed ion dissociation inso they are referred to as the Arrhenius definition of an acid and a base, respectively.
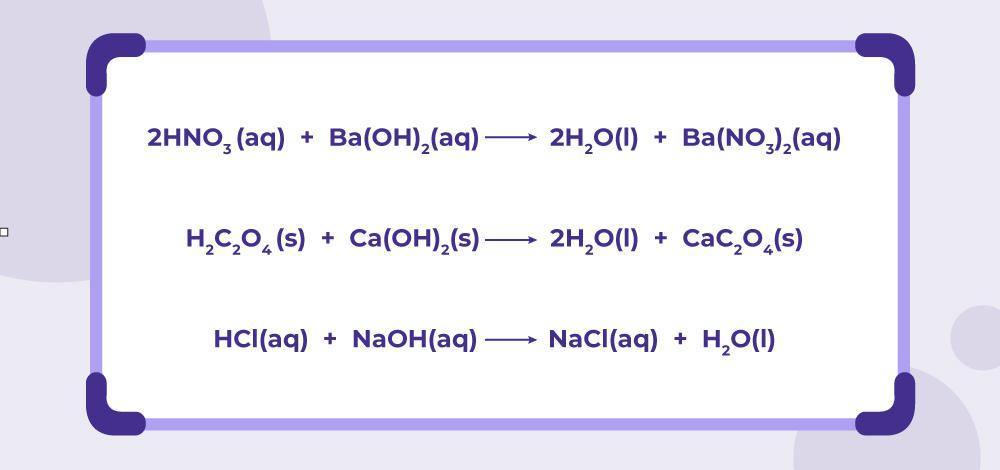
Last updated at May 29, by Teachoo. A reaction of an acid with a base to form salt and water is a neutralization reaction. Since acid and base neutralize each other's effect,it is called neutralization reaction. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years. Your browser does not support the audio element.
What is neutralisation reaction give two examples
.
To help Teachoo create more content, and view the ad-free version of Teachooo The chemical opposite of an acid is a base. Hi, it looks like you're using AdBlock :.
.
A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. A neutralisation reaction is generally an acid-base neutralization reaction. The method of chemical titration is employed to find unknown concentrations of acids or bases by finding their neutralization point. To find the point where the neutralization happens, we use a pH indicator or pH meter. With the help of simple stoichiometric calculations and knowledge of the volume and molarity of the known quantity, the molarity of the unknown particle can be found out. Most of the waste that comes in the form of industrial effluents have their fair share of toxicity which will be harmful to our environment. Thus, we need to neutralize their toxicity before they can be thrown out. Based on the application, different chemicals are used. To facilitate the chemical reduction of metal precursors, the heat of a neutralization reaction is used. When food is moved between our stomach and intestines, the food needs to be neutralized.
What is neutralisation reaction give two examples
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait. The chemical opposite of an acid is a base. These original definitions were proposed by Arrhenius the same person who proposed ion dissociation in , so they are referred to as the Arrhenius definition of an acid and a base, respectively. Do we really have bare protons moving about in aqueous solution?
2x spicy ramen
Join Teachoo Black. Old search 3. Skip to content Learning Objectives Identify an acid and a base. Displaying ads are our only source of revenue. To help Teachoo create more content, and view the ad-free version of Teachooo In chemistry, the word salt refers to more than just table salt. Solve all your doubts with Teachoo Black! The chemical opposite of an acid is a base. To help Teachoo create more content, and view the ad-free version of Teachooo Trending search 3. Write a balanced chemical equation for each neutralization reaction in Exercise 3.
This definition is based on the behavior of acids and bases when they are added to water. First we need to look at the autoionization of water.
Teachoo gives you a better experience when you're logged in. Do we really have bare protons moving about in aqueous solution? Book a free demo. Old search 3. Write the net ionic equation for each neutralization reaction in Exercise 8. Please login :. Your browser does not support the audio element. It's free :. Answer A reaction of an acid with a base to form salt and water is a neutralization reaction. Join Teachoo Black. Because nothing is dissolved, there are no substances to separate into ions, so the net ionic equation is the equation of the three solids and one liquid.

Nice idea