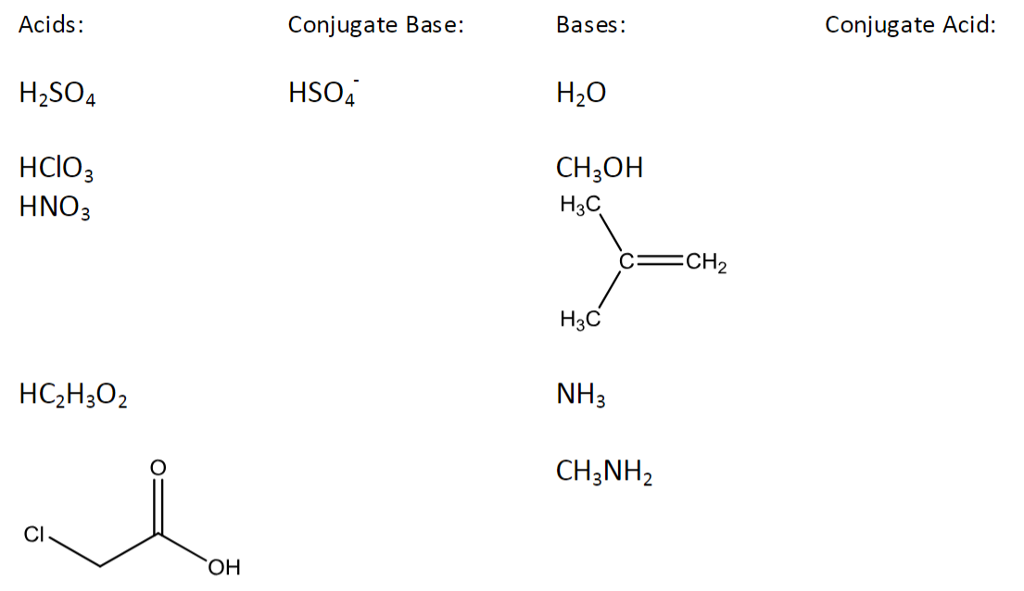
What is the conjugate base of h2so4
What is the conjugate of H 2 SO 4? A non-metallic element is converted into a compound X after a series of reactions.
Wiki User. They are the products of an acid-base reaction by the Bronsted-Lowry definition. Conjugate base. HSO4 -. Nope, itsHSO H2SO4 is already a strong acid. If you mean what is the conjugate base, then the answer is HSO
What is the conjugate base of h2so4
Identify the acid, base, conjugate acid and conjugate base in the following reaction. HSO4" aq …. A: Acid Base chemistry. Q: Which statement is true of this chemical equation? Q: acid, base, conjugate acid, and conjugate base. Q: Identify the acid, base, conjugate acid, and conjugate base in the following reactions. A: According to Bronsted-Lowry concept an acid is a proton donor and a base is a proton acceptor. Q: What is the conjugate base of phosphoric acid? A: The acid given is phosphoric acid i. Q: Which of the following acids would have the strongest conjugate base?
A: An acid donates a proton to form a conjugate species.
.
See this Socratic answer. A conjugate base contains one less H atom and one more - charge than the acid that formed it. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions. It has one less H atom and one more — charge. Acid strength is determined by the amount of that acid that actually ionizes. All other acids are weak acids and ionize to a much lesser amount. All acids have a conjugate base.
What is the conjugate base of h2so4
Through examples found in the sections on acids and bases proton-transfer processes are broken into two hypothetical steps: 1 donation of a proton by an acid, and 2 acceptance of a proton by a base. Water served as the base in the acid example and as the acid in the base example [ amphiprotic ]. The hypothetical steps are useful because they make it easy to see what species is left after an acid donated a proton and what species is formed when a base accepted a proton. We shall use hypothetical steps or half-equations in this section, but you should bear in mind that free protons never actually exist in aqueous solution. Suppose we first consider a weak acid , the ammonium ion.
Alica schmidt
H,0 is a base…. Zumdahl, Donald J. John C. Ka for… A: The more ka value of acid the more acidic and the conjugatebase of that strong acid is weakest…. Q: Which of the following pairs of species is not a conjugate acid—base pair? Study now See answers 9. A strong acid will form a weak…. H2SO4 is already a strong acid. Q: 1 Complete the following reactions and identify the acid, base, conjugate acid and conjugate base. Q: Which of the following acids would have the strongest conjugate base? H2C5H6O4 glutaric acid d.
On the other hand, a conjugate base is what remains after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a substance formed by the removal of a proton from an acid, as it can gain a hydrogen ion in the reverse reaction. A proton is a subatomic particle in the nucleus with a unit positive electrical charge.
H2SO4 is already a strong acid. Knowledge Booster. Find more answers Ask your question. H2C5H6O4 glutaric acid d. HCO,- 3. Arrhenius Acid Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Co,2- 2. What is conjugate acid and conjugate base? What is the formula of the conjugate acid for base SO4? A: The base which does not dissociate completely in aqueous solution is called weak base. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Standard XII Chemistry.

Excuse, I have thought and have removed this phrase
I consider, that you are not right. I am assured. I can defend the position. Write to me in PM.