What is the difference between ionic and covalent bonds quizlet
For more option use Advanced Search.
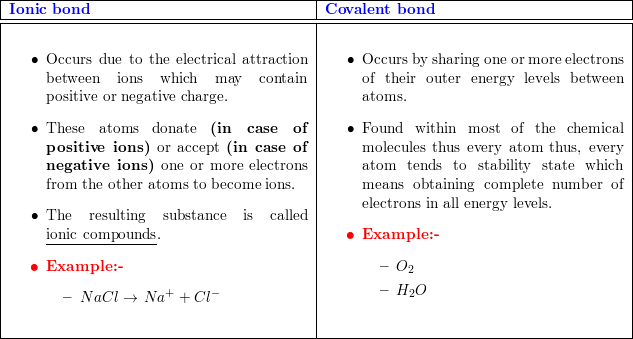
There are two types of atomic bonds - ionic bonds and covalent bonds. They differ in their structure and properties. Covalent bonds consist of pairs of electrons shared by two atoms, and bind the atoms in a fixed orientation. Whether two atoms can form a covalent bond depends upon their electronegativity i. This results in a positively charged ion cation and negatively charged ion anion. The bond between these two ions is called an ionic bond. The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the "sharing" is so unequal that an electron from atom A is completely lost to atom B, resulting in a pair of ions.
What is the difference between ionic and covalent bonds quizlet
.
Each nitrogen atom is able to share three electrons for a total of six shared electrons in the N 2 molecule Fig.
.
Ionic and covalent bonds are the two main types of chemical bonding. A chemical bond is a link formed between two or more atoms or ions. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Here is an explanation of the difference between ionic and covalent bonds, examples of each bond type, and a look at how to tell which type of bond will form. In an ionic bond, one atom donates an electron to another atom. This stabilizes both atoms. Because one atom essentially gains an electron and the other loses it, an ionic bond is polar. In other words, one atom in the bond has a positive charge, while the other has a negative charge.
What is the difference between ionic and covalent bonds quizlet
It has long been known that pure carbon occurs in different forms allotropes including graphite and diamonds. This molecule was named after the architect and inventor R. Buckminster Fuller — , whose signature architectural design was the geodesic dome, characterized by a lattice shell structure supporting a spherical surface. Experimental evidence revealed the formula, C 60 , and then scientists determined how 60 carbon atoms could form one symmetric, stable molecule.
Cuartos de azotea en renta
Ionic and covalent compounds also differ in what happens when they are placed in water, a common solvent. Each nitrogen atom is able to share three electrons for a total of six shared electrons in the N 2 molecule Fig. Covalent compounds tend to be soft, and have relatively low melting and boiling points. This document may be freely reproduced and distributed for non-profit educational purposes. For example, let us consider a Methane molecule i. Covalent Compounds. For example, when a crystal of sodium chloride is put into water, it may seem as though the crystal simply disappears. In a covalent bond, the atoms bond by sharing electrons. Carbon is represented with four unpaired electrons see Fig. As water evaporates, the salt solution becomes more and more concentrated. These are mostly gaseous and even a slight negative or positive charge at opposite ends of a covalent bond gives them molecular polarity. Covalent bonding is a form of chemical bonding between two non metallic atoms which is characterized by the sharing of pairs of electrons between atoms and other covalent bonds.
In BIS2A, we focus primarily on three different bond types: ionic bonds , covalent bonds , and hydrogen bonds.
Occurs between Two non-metals One metal and one non-metal Electrons Electrons are shared in covalent bonds. The bones represent one of their electrons. Terms of use Privacy policy. Covalent bonds have a definite and predictable shape and have low melting and boiling points. This document may be freely reproduced and distributed for non-profit educational purposes. Water, a liquid composed of covalently bonded molecules, can also be used as a test substance for other ionic and covalently compounds. Hope it helps. Three things are actually happening. This really helped me on my Covalent and Ionic Bonding worksheet! Covalent bonds are formed as a result of the sharing of one or more pairs of bonding electrons. In an ionic bond, the atoms are bound together by the electrostatic forces in the attraction between ions of opposite charge. Most of them are soluble in water but insoluble in non-polar solvents.

0 thoughts on “What is the difference between ionic and covalent bonds quizlet”