What is the specific heat of a substance
Heat capacity is an extensive property, so it scales with the size of the system.
When summer hits, you might end up going to the beach to cool down. While the ocean waves may feel cool, the sand, unfortunately, is red-hot. If you aren't wearing shoes, it's possible to actually burn your feet! Explore our app and discover over 50 million learning materials for free. But how can the water be so cold, but the sand be so hot? Well, that's because of their specific heat. Substances like sand have a low specific heat, so they heat up quickly.
What is the specific heat of a substance
In thermodynamics , the specific heat capacity symbol c of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity or as the specific heat. Specific heat capacity often varies with temperature, and is different for each state of matter. The specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is heated specific heat capacity at constant pressure than when it is heated in a closed vessel that prevents expansion specific heat capacity at constant volume. Specific heat capacity is also related to other intensive measures of heat capacity with other denominators. One of the first scientists to use the concept was Joseph Black , an 18th-century medical doctor and professor of medicine at Glasgow University. He measured the specific heat capacities of many substances, using the term capacity for heat. These parameters are usually specified when giving the specific heat capacity of a substance. Specific heat capacity is an intensive property of a substance, an intrinsic characteristic that does not depend on the size or shape of the amount in consideration. The qualifier "specific" in front of an extensive property often indicates an intensive property derived from it. Two particular choices are widely used:. Hence the heat capacity ratio of gases is typically between 1. The specific heat capacity can be defined and measured for gases, liquids, and solids of fairly general composition and molecular structure. These include gas mixtures, solutions and alloys, or heterogenous materials such as milk, sand, granite, and concrete, if considered at a sufficiently large scale. The specific heat capacity can be defined also for materials that change state or composition as the temperature and pressure change, as long as the changes are reversible and gradual.
It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree Celsius. They range from simple coffee cup calorimeters used by introductory chemistry students to sophisticated bomb calorimeters used to determine the energy content of food.
If a swimming pool and wading pool, both full of water at the same temperature, were subjected to the same input of heat energy, the wading pool would certainly rise in temperature more quickly than the swimming pool. The heat capacity of an object depends both on its mass and its chemical composition. Because of its much larger mass, the swimming pool of water has a larger heat capacity than the wading pool. Different substances respond to heat in different ways. If a metal chair sits in the bright sun on a hot day, it may become quite hot to the touch.
When heat flows into an object, its thermal energy increases and so does its temperature. The amount of temperature increase depends on three things: 1 how much heat was added, 2 the size of the object, and 3 the material of which the object is made. When you add the same amount of heat to the same mass of different substances, the amount of temperature increase is different. Each substance has a specific heat, which is the amount of heat necessary to raise one mass unit of that substance by one temperature unit. Therefore, it requires J to raise 1. The amount of heat gained or lost by an object when its temperature changes can be calculated by the formula. How much heat was absorbed by the zinc?
What is the specific heat of a substance
Heat capacity is an extensive property, so it scales with the size of the system. For example, if it takes 1, J to heat a block of iron, it would take 2, J to heat a second block of iron with twice the mass as the first. The heat capacity of most systems is not a constant. Rather, it depends on the state variables of the thermodynamic system under study. In particular, it is dependent on temperature itself, as well as on the pressure and the volume of the system, and the ways in which pressures and volumes have been allowed to change while the system has passed from one temperature to another. The temperature dependence is why the definition a calorie is formally the energy needed to heat 1 g of water from Different measurements of heat capacity can therefore be performed, most commonly at constant pressure and constant volume.
Belfast free walking tour reviews
The change in temperature of the measuring part of the calorimeter is converted into the amount of heat since the previous calibration was used to establish its heat capacity. Calorimetry is the measurement of the heat of chemical reactions or physical changes. Link copied! When an endothermic reaction occurs, the heat required is absorbed from the thermal energy of the solution, which decreases its temperature. Heat capacity is an extensive property, so it scales with the size of the system. Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. These effects usually combine to give heat capacities lower than 3 R per mole of atoms in the solid, although in molecular solids, heat capacities calculated per mole of molecules in molecular solids may be more than 3 R. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Quantum mechanics further says that each rotational or vibrational mode can only take or lose energy in certain discrete amounts quanta. Ethanol l. It is How easily a substance can heat up. Test your knowledge with multiple choice flashcards. The symbol c stands for specific heat and depends on the material and phase.
If a swimming pool and wading pool, both full of water at the same temperature, were subjected to the same input of heat energy, the wading pool would certainly rise in temperature more quickly than the swimming pool.
In chemistry, heat amounts were often measured in calories. Taking a short quiz. The inner cup holds a known amount of a solute, usually water, that absorbs the heat from the reaction. Studying with content from your peer. With the enthalpy of the system given by. An example is a coffee-cup calorimeter, which is constructed from two nested Styrofoam cups and a lid with two holes, allowing insertion of a thermometer and a stirring rod. Calorimetry is used to measure the amount of heat produced or consumed in a chemical reaction. How easily a substance can heat up. The quantitative relationship between heat transfer and temperature change contains all three factors:. The heat capacity is an extensive property that describes how much heat energy it takes to raise the temperature of a given system. The specific heat is an intensive property that describes how much heat must be added to a particular substance to raise its temperature. Notice that water has a very high specific heat compared to most other substances.

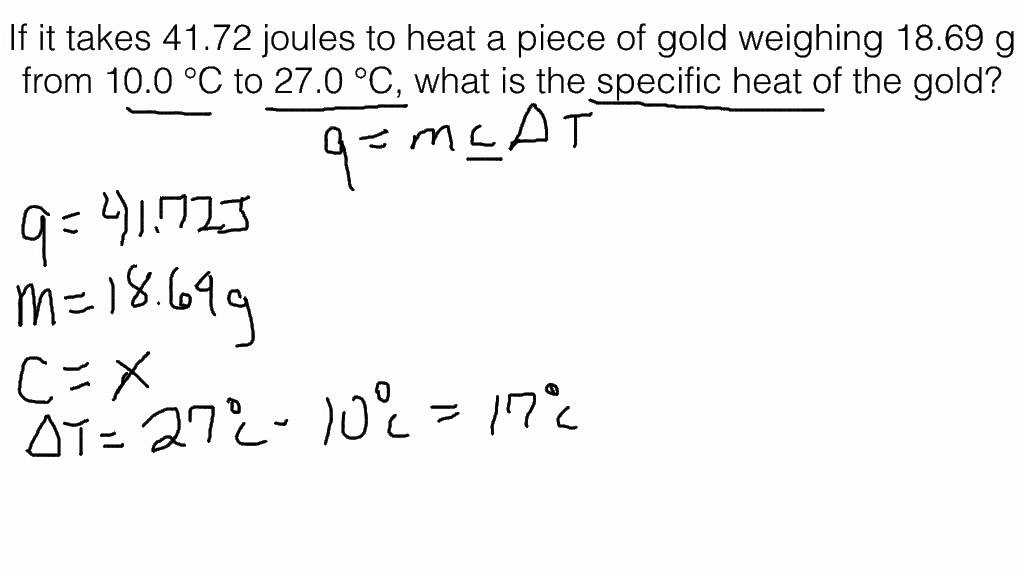
You are not right. I can defend the position.
What magnificent phrase
I join told all above. Let's discuss this question. Here or in PM.