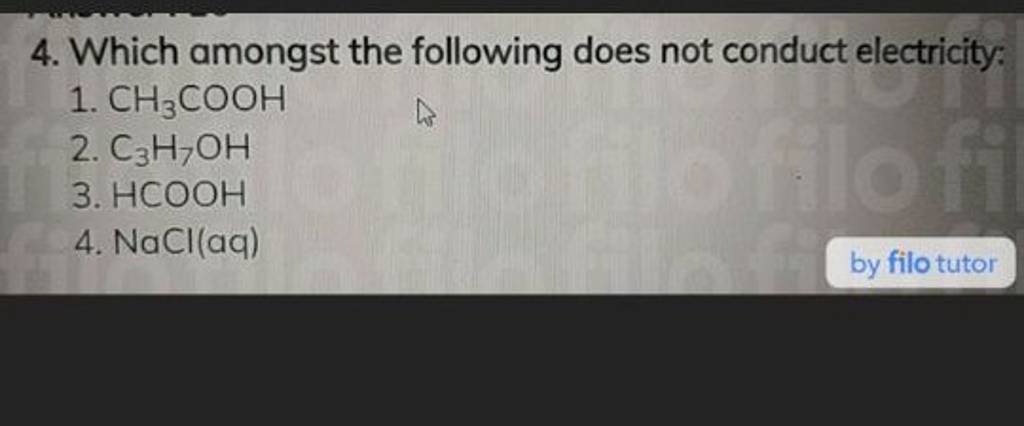
Which of the following does not conduct electricity
Last updated on Jan 24, The last date for application correction is 29th February
Additional Information. Last updated on Mar 7, Get Started. SSC Exams. Banking Exams.
Which of the following does not conduct electricity
Lime juice. Salt solution. Pure water. Which of the following will not conduct electricity? Which of the following objects can conduct electricity? An experiment was performed to test the electrical conductivity of some substances as shown in figure below: The soluton of which of the following will not conduct electricity? Which of the following liquids can conduct electricity in pure state? Which of the following can conduct electricity in? Which amongst the following will not conduct electricity? Which of the following does not conduct current in aqueous solution. Which of following will not conduct electricity? Which of the following 1M conducts more electricity? Filament of the bulb is made of. Which of these will generate electric current when kept in sunlight Which of the following does not conduct electricity?
NBE Junior Assistant. Visva Bharati MTS. CG Lab Attendant.
.
In association with Nuffield Foundation. In this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. This experiment enables students to distinguish between electrolytes and non-electrolytes, and to verify that covalent substances never conduct electricity even when liquefied, whereas ionic compounds conduct when molten. The practical works well as a class experiment, with students working in groups of two to three. There will not be time to investigate all the substances, so each group could be assigned three or four of these, and the results pooled at the end. The apparatus required for testing the conductivity of different substances when solid and molten. The covalent solids only need to be heated for a short time for melting to take place. The students should be warned about what to do if this happens eg cover with a damp cloth. The experiments should be done in a well-ventilated laboratory. It may be helpful to reserve a crucible for each of the powdered compounds, while having one or two others that can be heated.
Which of the following does not conduct electricity
Some substances, such as metals and salty water, allow charges to move through them with relative ease. Some of the electrons in metals and similar conductors are not bound to individual atoms or sites in the material. These free electrons can move through the material much as air moves through loose sand. Any substance that has free electrons and allows charge to move relatively freely through it is called a conductor. The moving electrons may collide with fixed atoms and molecules, losing some energy, but they can move in a conductor. Superconductors allow the movement of charge without any loss of energy.
Winter tires costco price
UK Police Constable. CISF Driver. UP PGT. Bihar Police Forest Guard. Indian Bank SO. Maharashtra Zilla Parishad JE. Gujarat Metro Maintainer. Teaching Exams. MP Mahila Supervisor. Gujarat Metro JE. Rajasthan High Court Clerk. MP Vyapam Group 5. Which of these waves is a Electromagnetic waves?
What makes a material a conductor or an insulator? Simply put, electrical conductors are materials that conduct electricity and insulators are materials that do not. Whether a substance conducts electricity is determined by how easily electrons move through it.
NFC Stipendiary Trainee. Kerosene oil is an example of RBI Security Guard. Telangana High Court Record Assistant. Was this answer helpful? Gujarat Metro Maintainer. Why is the metal spring used in electric torch? Kerala Beat Forest Officer. AP Police SI. KSP Constable.

Yes, really. And I have faced it.
I am assured, that you are not right.