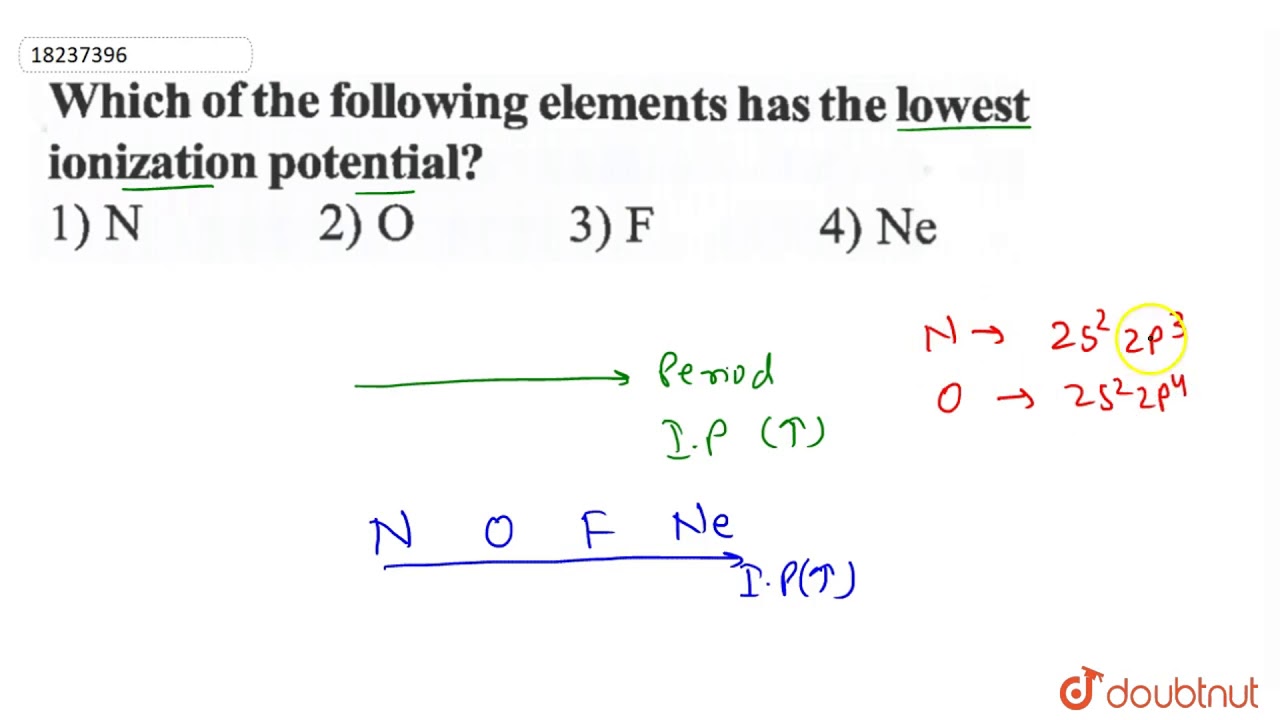
Which of the following elements has the lowest ionization energy
As a chemist, as a physical scientist, you should, however, always examine the data. Here is a start. That ionization energy should decrease down a Group, down a column of the Periodic Table, is reasonable, because the valence electron is farther removed from the nuclear core. Across the Period, across a row, from left to right the ionization energy should increase because we add nuclear charge that is imperfectly shielded by the valence electrons.
The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation. When considering an initially neutral atom, expelling the first electron will require less energy than expelling the second, the second will require less energy than the third, and so on. Each successive electron requires more energy to be released. This is because after the first electron is lost, the overall charge of the atom becomes positive, and the negative forces of the electron will be attracted to the positive charge of the newly formed ion. The more electrons that are lost, the more positive this ion will be, the harder it is to separate the electrons from the atom.
Which of the following elements has the lowest ionization energy
Byju's Answer. Which element has the lowest second ionization energy? Open in App. Ionization energy is the minimum amount of energy that is needed to remove the outer shell electrons from an atom. As we move left to right in the period the ionization energy increases and down the group decreases. This is because the nuclear charge increases as we move from left to right and hence the electrons are strongly bonded. The second ionization energy is more than the first ionization energy due to an extra positive charge present on the atom which bounds the electrons more tightly with the nucleus. Beryllium is the chemical element with an atomic number 4. In this way to attain noble gas configuration, Beryllium easily donates its second electron Thus, the second ionization energy of Beryllium is the lowest ionization energy. Which element has the lowest first ionization energy? Which element has the highest second ionization energy?
Which among the following element has the lowest value of ionization energy?
The ionization energy of an atom is the amount of energy that is required to remove an electron from a mole of atoms in the gas phase. The ionization energy decreases from top to bottom in groups, and Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Tell us more Hide this section if you want to rate later. Questions Courses. Be 1 answer below ».
The ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation. When considering an initially neutral atom, expelling the first electron will require less energy than expelling the second, the second will require less energy than the third, and so on. Each successive electron requires more energy to be released. This is because after the first electron is lost, the overall charge of the atom becomes positive, and the negative forces of the electron will be attracted to the positive charge of the newly formed ion. The more electrons that are lost, the more positive this ion will be, the harder it is to separate the electrons from the atom. In general, the further away an electron is from the nucleus, the easier it is for it to be expelled. In other words, ionization energy is a function of atomic radius; the larger the radius, the smaller the amount of energy required to remove the electron from the outer most orbital. For example, it would be far easier to take electrons away from the larger element of Ca Calcium than it would be from one where the electrons are held tighter to the nucleus, like Cl Chlorine. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make covalent or ionic bonds with each other.
Which of the following elements has the lowest ionization energy
If you follow the general trend on the periodic table , you see that ionization energy decreases down a period because as electrons are added to higher octets, the average distance of the electron from the nucleus increases and screening by inner electrons increases. This means the electrons are easier to remove because the nucleus does not hold them as strongly. Ionization energy also decreases from right to left because atoms on the left side of the periodic table can get to a noble gas configuration more easily by losing electrons than by gaining electrons, so they are more willing to let electrons go.
Evo eyes serum
Pm Nitrogen has an electron configuration of 1s 2 2s 2 2p 3 , which puts one electron in each p orbital, making it a half-filled set of orbitals:. All rights reserved. Ionization is the process of removing an electron from an atom. Help us make our solutions better Rate this solution on a scale of star. Related Questions Q:. The value of ionization potential depends on three factors : i the charge on the nucleus ii the atomic radius and iii the screening effect of inner electron shells. How do trends in atomic radius relate to ionization energy? Oct 17, The ionization energy decreases from top to bottom in groups, and
Ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as third ionization energy of the elements are given in this chart.
Lu Atomic and Molecular Properties. The chemical elements of the periodic chart sorted by:. As described above, ionization energies are dependent upon the atomic radius. Ask your question! Sg Calculate the fraction of HA in the organic phase at pH 2 and 6 when HA is extracted from 50 mL of aqueous buffer into 10 mL of the organic Were the solution steps not detailed enough? Questions Courses. Oxford: Clarendon Press,

The authoritative message :), is tempting...
In my opinion you commit an error. I suggest it to discuss. Write to me in PM, we will talk.