B lewis structure
A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule.
Together the three resonance structures suggest partial double-bond character in the Be-X bond, which results in an intermediate bond length between a single and double bond. There are issues with each of these resonance structures. The structure in the middle is a mix of these problems. None of these situations is ideal according to Lewis theory. In contrast to BeF 2 , solid BeCl 2 is a 1-dimensional polymer consisting of edge-shared tetrahedral. In the gas phase, BeCl 2 exists as a dimer with two chlorine atoms bridging two Be atoms. In the dimer, the Be atoms are 3-coordinate.
B lewis structure
Dec 15, Chemistry. This structure is used to predict the chemical behavior of atoms and molecules. But how to draw the lewis dot structure? Lewis dot structure. Boron belongs to group 13 of the periodic table, and its electronic configuration is 2,3. This means it has three electrons in its valence shell, two in the 2s orbital and one in the 2p orbital. A Lewis dot structure is a graphic representation of the electron distribution around atoms. This also predicts the type and number of bonds formed around an atom. It is also possible to predict the geometry of a molecule by using a lewis dot structure. Here are some steps on how to draw lewis dot diagram of a moleculeto follow while drawing a lewis dot structure of any molecule:. Sum up the valence electrons in the molecule from all the atoms. Covalent bonds form when an electron from every atom forms an electron pair. Step 2 indicates the number of required electrons, while Step 1 indicates the number of electrons you have.
Miessler; D. Triplet Singlet Exchange-coupled.
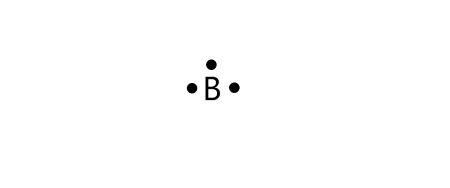
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons as shown in Figure Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:.
B lewis structure
We know that the formation of covalent bonds is achieved by an overlap of orbitals between two atoms that share a pair of valence electrons. The bonds are most often shown with either two dots or a line, and these representations are called Lewis symbols or Lewis structures. So the bonds can be represented either with a line or a pair of electrons two dots and therefore, we can say that Lewis structures are electron-dot representations for molecules. Once we know this concept, it is relatively easy to interpret the structure. However, coming up with a reasonable Lewis structure from a formula is not as obvious.
Walgreens free photo coupon code
We examined XeF 4 earlier. In this case, there is no central atom, so we distribute the electrons around both atoms. Main article: Resonance structure. The total number of electrons does not change. But how to draw the lewis dot structure? Carbon tetrachloride was formerly used in fire extinguishers for electrical fires. Place all remaining electrons on the central atom. Both of these gases also cause problems: CO is toxic and CO 2 has been implicated in global climate change. Lewis Structures We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions. Boron B and fluorine F have atomic numbers of 5 and 9, respectively. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions.
However, the B—F bonds are slightly shorter than what is actually expected for B—F single bonds, indicating that some double bond character is found in the actual molecule. Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol? Next: Formal Charges and Resonance. Figure 1. Write the Lewis structure for the diatomic molecule P 2 , an unstable form of phosphorus found in high-temperature phosphorus vapor. No Lewis structure is complete without the formal charges. Non-valence electrons are not represented in Lewis structures. Hydrogen atoms bonded to carbon are not shown—they can be inferred by counting the number of bonds to a particular carbon atom—each carbon is assumed to have four bonds in total, so any bonds not shown are, by implication, to hydrogen atoms. See these examples: For more complicated molecules and molecular ions, it is helpful to follow the step-by-step procedure outlined here: Determine the total number of valence outer shell electrons. Then, write the Lewis symbol for the common ion formed from each atom:. N 5 O x 3 18 charge 1 24 Draw a skeletal structure for the molecule which connects all atoms using only single bonds. The Lewis Dot Structure allows visibility of all electrons and determines if an atom follows the octet rule. Fullerene Chemistry.

What good phrase