Ca oh 2 aq
The solubility of calcium hydroxide and the aqueous speciation ca oh 2 aq Ca II in alkaline medium at various temperatures and background electrolyte concentrations were characterized by solubility measurements applying ICP-OES and potentiometric detection methods. The most important implication of this model is that the total concentration of the dissolved calcium II cannot be decreased below ca. This statement was further validated via precipitation titrations. The standard enthalpies and entropies of the reactions were also calculated from temperature-dependent solubility measurements, ca oh 2 aq.
There is no need to include water in the ionization equation, you just need to include the states in your equation:. You may want to write an equation corresponding to the hydroxide displacement in water The Grotthuss mechanism but it is not a measurable process since the reactants and products are the same. How do you write the ionization equation for calcium hydroxide: Ca OH 2? Kathryn Bell. Nov 21, Related questions What ions are present in solutions of sodium hydroxide? Why is orange juice considered a solution?
Ca oh 2 aq
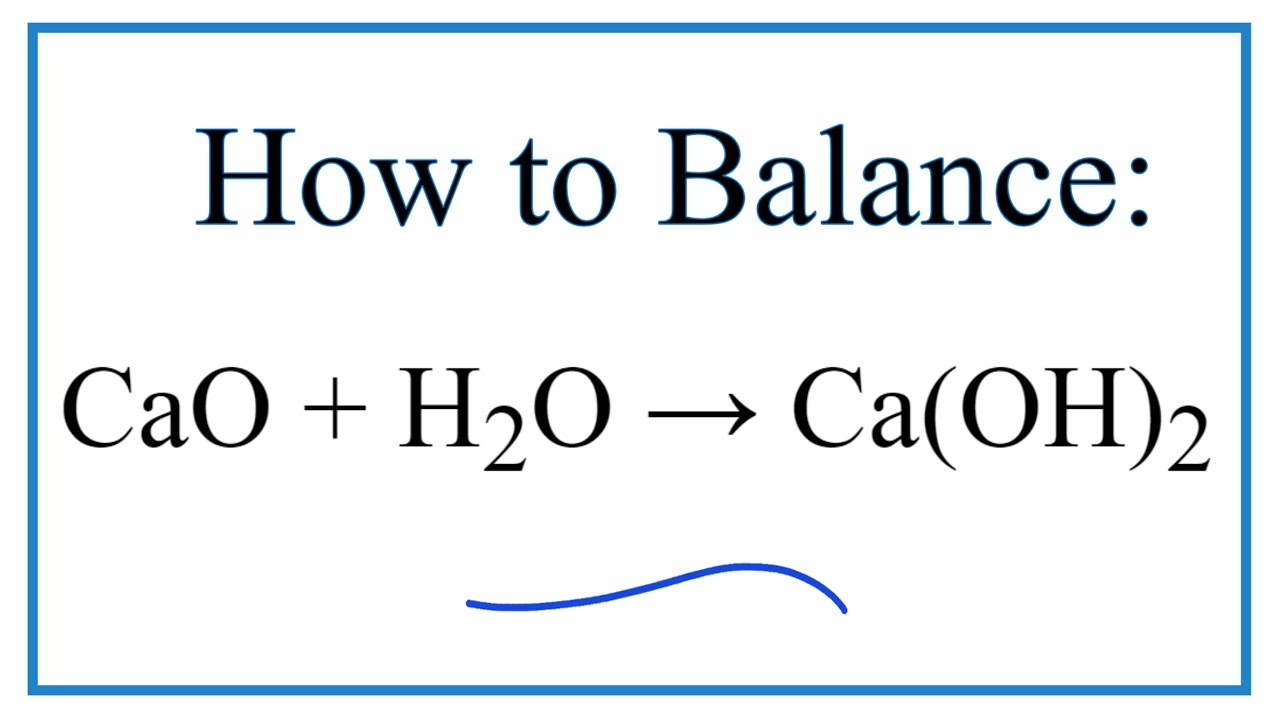
Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca OH 2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca OH 2 has a colourless appearance in its crystalline form. The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide also known as quick lime. The chemical reaction between sodium hydroxide and calcium chloride dissolved in water aqueous CaCl 2 also yields this compound. The structure of a Ca OH 2 molecule is illustrated below. Unprotected exposure to this compound can prove dangerous to humans, leading to irritation of the skin and chemical burns. Exposure to concentrated Ca OH 2 can lead to lung damage and even blindness. Calcium hydroxide is a white powder which does not have any characteristic odour. It is used in industrial settings such as the treatment of waste, the manufacturing of paper, building and processing of food. There are also several medical and dental applications of this compound. For instance, fillings used in root canal treatments often contain calcium hydroxide. Calcium hydroxide, also known as slaked lime with the chemical formula Ca OH 2 is a source of hydroxide ions when dissolved in aqueous solutions.
Share Share Share Call Us. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed with water. It has many names including hydrated lime , caustic lime , builders' lime , slaked lime , cal , and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E Limewater , also called milk of lime , is the common name for a saturated solution of calcium hydroxide.
There is no need to include water in the ionization equation, you just need to include the states in your equation:. You may want to write an equation corresponding to the hydroxide displacement in water The Grotthuss mechanism but it is not a measurable process since the reactants and products are the same. How do you write the ionization equation for calcium hydroxide: Ca OH 2? Kathryn Bell. Nov 21, Related questions What ions are present in solutions of sodium hydroxide? Why is orange juice considered a solution? Doesn't it have pulp in it so it's a suspension?
Ca oh 2 aq
For single-replacement and double-replacement reactions, many of the reactions included ionic compounds—compounds between metals and nonmetals, or compounds that contained recognizable polyatomic ions. Now, we take a closer look at reactions that include ionic compounds. One important aspect about ionic compounds that differs from molecular compounds has to do with dissolution in a liquid, such as water. When molecular compounds, such as sugar, dissolve in water, the individual molecules drift apart from each other. When ionic compounds dissolve, the ions physically separate from each other. We can use a chemical equation to represent this process—for example, with NaCl:. This process is called dissociation; we say that the ions dissociate. All ionic compounds that dissolve behave this way. This behavior was first suggested by the Swedish chemist Svante August Arrhenius [—] as part of his PhD dissertation in Interestingly, his PhD examination team had a hard time believing that ionic compounds would behave like this, so they gave Arrhenius a barely passing grade.
I hate google drive
What are spectator ions? This statement was further validated via precipitation titrations. Cited by. Retrieved 12 May Sc OH 3. View Result. It forms a fluffy charged solid that aids in the removal of smaller particles from water, resulting in a clearer product. Am OH 3. Impact of this question views around the world. How can I calculate the moles of ions in solutions? It has also been known to arise in burning coal dumps. First published 02 May Agriculture Marketing Services. Gd OH 3.
Calcium hydroxide, Ca OH 2 , forms colorless crystals resulting in white powder and is obtained by mixing calcium oxide with water calcium hydroxide is also called slaked lime. Calcium hydroxide is produced commerically in enormous quantities by thermal decomposition of limestone and subsequent exothermic reaction of the calcium oxide with water:. The exothermic reaction with water yields enough energy to evaporate the water.
See all questions in Reactions Between Ions in Solutions. Lu OH 3. How can I calculate the moles of ions in solutions? D Y. Inorganic Chemistry. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca OH 2. Tc OH 4. Magnesium Oxide Formula. Please enable JavaScript to access the full features of the site or access our non-JavaScript page. What are spectator ions? Geochimica et Cosmochimica Acta. Gd OH 3.

It is not pleasant to me.