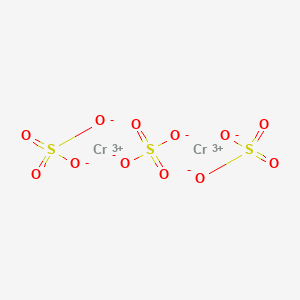
Cr2 so4 3
Then, lookup atomic weights for each element in periodic table : Cr: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students, cr2 so4 3. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy.
Molar mass of Cr 2 SO 4 3 is Then, lookup atomic weights for each element in periodic table : Cr: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Cr2 so4 3
With finding oxidation numbers , you have to know the common oxidation states of an element. There might be some little algebra involved. Since sulfur and oxygen atoms are enclosed in a parenthesis, this means that they are in covalent bond with each other and are therefore "sharing" electrons. So if the oxygen is negatively charged, the sulfur must be positively charged. What is the oxidation number of Cr in Cr2 SO4 3? Chemistry Electrochemistry Oxidation Numbers. Nikka C. Oct 25, Explanation: With finding oxidation numbers , you have to know the common oxidation states of an element. Related questions How do oxidation numbers relate to electron configuration? How do oxidation numbers relate to valence electrons?
Chemical forum.
Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather. A variety of other chromium III sulfates are known, but also contain hydroxide or oxide ligands. Other chromium III hydroxides have been reported.
This page looks at some aspects of chromium chemistry. It includes: reactions of chromium III ions in solution summarised from elsewhere on the site ; the interconversion of the various oxidation states of chromium; the chromate VI -dichromate VI equilibrium; and the use of dichromate VI ions as an oxidizing agent including titrations. The ion reacts with water molecules in the solution. A hydrogen ion is lost from one of the ligand water molecules:. The complex ion is acting as an acid by donating a hydrogen ion to water molecules in the solution. The water is, of course, acting as a base by accepting the hydrogen ion.
Cr2 so4 3
What is this information? Department of Transportation hazard labels, and a general description of the chemical. Dark green to violet crystalline material. Used in paints and inks, ceramics, and in textile dyeing. It is noncombustible. The primary hazard of this material is the potential for environmental damage if released. Immediate steps should be taken to limit its spread to the environment. The Hazard fields include special hazard alerts air and water reactions, fire hazards, health hazards, a reactivity profile, and details about reactive groups assignments and potentially incompatible absorbents. Special Hazards of Combustion Products: Decomposes to chromic acid when heated. USCG,
Fotos de mujeres en el espejo
Weights of atoms and isotopes are from NIST article. Find atomic masses: look up the atomic masses of each element present in the compound. In other projects. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Impact of this question views around the world. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. How do oxidation numbers vary with the periodic table? Gas laws. Nikka C. Contact us. One mole contains exactly 6. What is the oxidation number for oxygen?
Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water.
Chemistry tools. Chemistry tools. Contact us. Common compound names. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. CO 2 has one carbon atom and two oxygen atoms. Unit converters. Related questions How do oxidation numbers relate to electron configuration? Nikka C.

Bravo, what words..., a remarkable idea