Daniell cell class 12
Byju's Answer. Explain Daniel cell with cell diagram representation and process taking place in the cell.
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathode , respectively. Both metals are submerged in the corresponding salt solutions. A Daniell cell is a device that transforms chemical energy released by redox reactions into electrical energy. It has a 1. Zinc Zn , which serves as the anode in a Daniell Cell, and Copper Cu , which serves as the cathode, are the two different metals in use.
Daniell cell class 12
Padma Priya and Laboratory Assistant, Mr. Suresh for helping me complete this project. I thank the principal of our school, Mrs. T for providing us with a well equipped laboratory for carrying out our experiments. I would like to thank my parents for supporting my work on this project. One of their oldest and most simple incarnation was the Daniell cell. If u have ever wondered how a cell works, the Daniell cell is the best way to practically experience and understand it. This project looks into how a Daniell cell works and how the change in ratio and purity of the substances used would affect the output of the cell. This model of a Daniell cell is made to get a closer look and gain more understanding on how they work. Table of contents 1. It consists of a copper pot filled with a solution of copper sulfate, in which an unglazed earthenware container filled with sulfuric acid and zinc electrode is immersed. The original design consisted of a 3.
One must notice that the positively charged copper ions always move towards the anode that is positively charged. Out in.
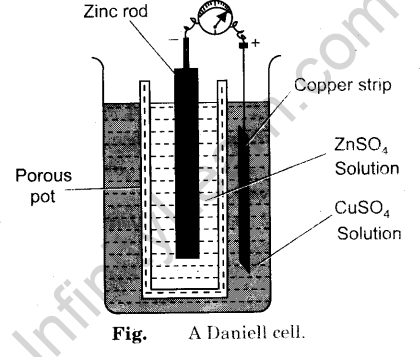
In this article, you will learn in detail about Daniell cell, its definition, construction, the chemical reaction involved, and its applications. Read on for more. A Daniell cell is a type of electrochemical cell that consists of a copper pot that is filled with a solution of copper II sulphate. An unglazed earthenware is immersed in this solution containing sulphuric acid and a zinc electrode. The Daniell cell was invented, while the chemist was seeking a way to eliminate the hydrogen bubble issue found in the voltaic pile.
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with. The greatest example of a galvanic cell that turns chemical energy into electrical energy is a Daniell cell. The Daniell cell is made up of two electrodes made of different metals, Zn and Cu, which are in contact with a solution of their respective ions, zinc sulphate and copper sulphate. Register Now. A conventional galvanic cell, it is meant to generate an electric current by using the spontaneous redox reaction between zinc and cupric ions. A copper vessel makes up this cell. In this case, a saturated CuSO 4 solution is used as a depolarizer and diluent.
Daniell cell class 12
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathode , respectively. Both metals are submerged in the corresponding salt solutions.
Laser tag brainerd mn
Assignment 6 Assignment 6. The zinc metals break up into positive ions that combine with the sulphate ions to release more electrons. This flow of electrons from the cathode to the anode produces electricity. Thus, the above points can be summed up as: Voltaic or Galvanic cell consists of two half-cells. Select Grade 6th 7th 8th 9th 10th 11th 12th 12th Pass Please choose the valid grade. A copper disc perforated with numerous holes was placed across the cylinder recessed down from the top. This copper sulphate solution has a porous earthenware filled with dilute sulphuric acid and a zinc electrode. Zinc sulfate may be substituted for the sulfuric acid. The use of a porous barrier allows ions to pass through but keeps the solutions from mixing. A Typical Cell Structure An electrolyte, two electrodes, and an electrolytic cell make up an electrolytic cell a cathode and an anode. This provides an electrical current that illuminates the bulb. Bird's experiments with this cell were of some importance to the new discipline of electrometallurgy , but Bird himself did not pursue this field; his interest was in electrotherapy. In copper vessels there is a transparent layer all around just below the upper surface in which CuSO 4 crystals are kept in contact with CuSO 4 solution due to this the solution always remains saturated. When the zinc and copper electrodes are joined by wire, the following observations are made: There is a flow of electric current through the external circuit. This may help both parties to acquire more knowledge.
How does a Cell in a T.
Even after most telegraph lines started being powered by motor-generators, the gravity battery continued to be used in way stations to power the local circuit at least into the s. The ox-gullet acts as a porous membrane for the passage of ions. Deposition of copper, and other metals, had been previously noted, but always previously it had been metal on metal electrode. Even after most telegraph lines started being powered by motor-generators, the gravity battery continued to be used in way stations to power the local circuit at least into the s. Noting the keywords is important but you need to have a good vocabulary too. Both the Electrodes are dipped in their respective metal salt solutions. While electrolytic cells involve non-spontaneous reactions and therefore need an external electron source, such as a DC battery or an AC power source, galvanic cells get their energy from spontaneous redox reactions. At the anode, oxidation takes place and solid zinc converts into zinc ions. Image to be added soon. John Dancer , a Liverpool instrument maker, in was the first to take commercial advantage of the unique features of the Daniell cell for copper plating.

I can not participate now in discussion - it is very occupied. But I will return - I will necessarily write that I think on this question.
Do not take in a head!