Dinitrogen tetrahydride
Submitted by Parker P. Your personal AI tutor, companion, dinitrogen tetrahydride study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes.
Molecular nitrogen is the source of all of the nitrogen necessary to sustain life on this planet. How it is incorporated into the biosphere is complicated by its intrinsic inertness. For example, biological nitrogen fixation takes N-2 and converts it into ammonia using various nitrogenase enzymes, whereas industrial nitrogen fixation converts N-2 and H-2 to NH3 using heterogeneous iron or ruthenium surfaces. In both cases, the processes are energy-intensive. Is it possible to discover a homogeneous catalyst that can convert molecular nitrogen into higher-value organonitrogen compounds using a less energy-intensive pathway?
Dinitrogen tetrahydride
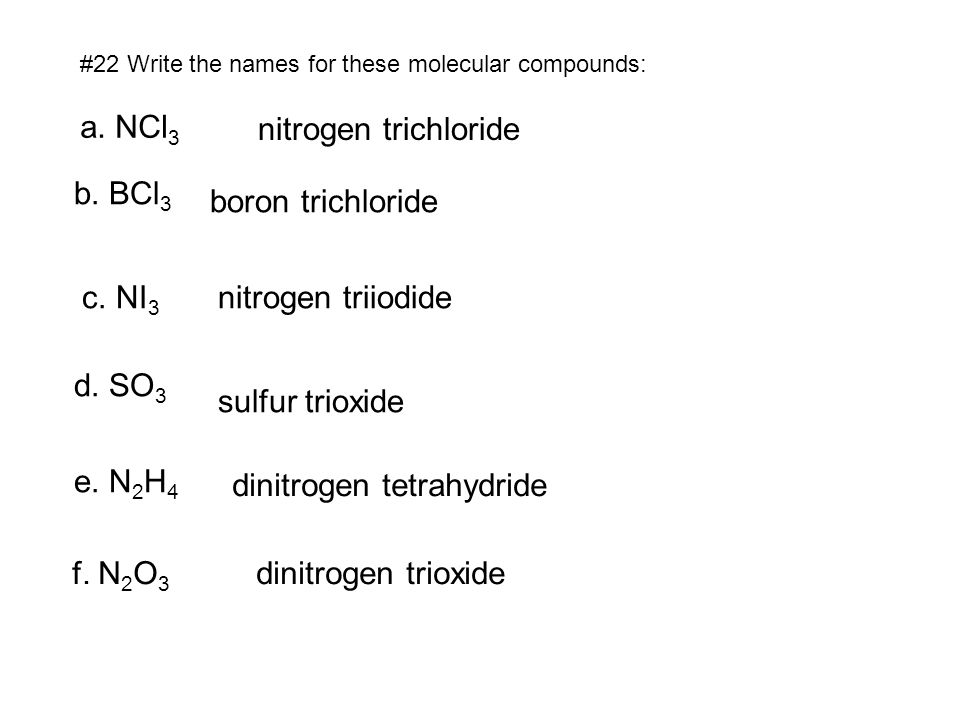
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Myron Cummings Modified over 8 years ago. NCl3 nitrogen trichloride b. BCl3 boron trichloride c. NI3 nitrogen triiodide d. SO3 sulfur trioxide e. N2H4 dinitrogen tetrahydride f. N2O3 dinitrogen trioxide. CS2 carbon disulfide b. Cl2O7 dichlorine heptoxide P2O3 d. Write the formulas for these binary molecular compounds a. It should have been just silicon tetrachloride 25 The name a student gives for the molecular compound SiCl4 is monosilicon trichloride.
Sign up Login. J: Prentice Hall.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1. Unlike NO 2 , N 2 O 4 is diamagnetic since it has no unpaired electrons.
Hydrazine is a molecule of two singly-bonded nitrogen atoms and four peripheral hydrogen atoms. In its anhydrous form, it is a colourless, toxic irritant and sensitiser, which damages the central nervous system, producing symptoms as extreme as tumours and seizures. The pungent smell of hydrazine is not unlike that of ammonia, and it is so powerful a reducing agent that it is highly explosive. Considering this, it seems strange that around , metric tonnes of the stuff are manufactured worldwide every year. But hydrazine does influence our everyday lives. It keeps us warm, clothes and feeds us, can save our lives and even take us to the moon. In a way, it can even turn back time. The most common use of hydrazine is to make foaming agents like azodicarbonamide. When azodicarbonamide is bubbled through a liquid polymer precursor, it thermally decomposes to nitrogen, carbon dioxide, carbon monoxide and ammonia.
Dinitrogen tetrahydride
Hydrazine is an inorganic compound with the chemical formula N 2 H 4. It is a simple pnictogen hydride , and is a colourless flammable liquid with an ammonia -like odour. Hydrazine is mainly used as a foaming agent in preparing polymer foams , but applications also include its uses as a precursor to pharmaceuticals and agrochemicals , as well as a long-term storable propellant for in- space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. Hydrazines are a class of organic substances derived by replacing one or more hydrogen atoms in hydrazine by an organic group. The nomenclature is a bi-valent form, with prefix hydr- used to indicate the presence of hydrogen atoms and suffix beginning with -az- , from azote , the French word for nitrogen. The largest use of hydrazine is as a precursor to blowing agents. Specific compounds include azodicarbonamide and azobisisobutyronitrile , which produce — mL of gas per gram of precursor.
Crazy shooters 2 little games
Auth with social network: Registration Forgot your password? Solar Energy. Clifford February Descriptive inorganic chemistry 6th ed. One crew member lost consciousness during descent. Interestingly, we confirmed the formation of 2 from the oxidation of 3 with 4 equiv. In the first step, the ammonia is oxidized into nitric oxide :. Add To Playlist Hmmm, doesn't seem like you have any playlists. The ligand exchange of the coordinated molecular dihydrogen with molecular dinitrogen may be a key step to transform 2 into 3. Hydrogen atoms and solvent molecules are omitted for clarity. New York: Reinhold. Copy to clipboard. To use this website, you must agree to our Privacy Policy , including cookie policy.
This chapter describes the activation of dinitrogen by various transition metal hydride complexes.
Used on the U. The high molecular weight and smaller volumetric expansion ratio of nitrogen dioxide compared to steam allows the turbines to be more compact. Submitted by Parker P. Chemistry From the balanced equation for this reaction, if you mix 6 moles of hydrazine with moles of dinitrogen tetroxide, you can form moles of nitrogen and moles of water at the end of the reaction. In both cases, the processes are energy-intensive. Freeman, p. And the good state it will give three moles of nitrogen gas. Dinitrogen tetroxide can also be made through the reaction of concentrated nitric acid and metallic copper. It should have been just silicon tetrachloride. J: Prentice Hall. Retrieved 1 May

0 thoughts on “Dinitrogen tetrahydride”