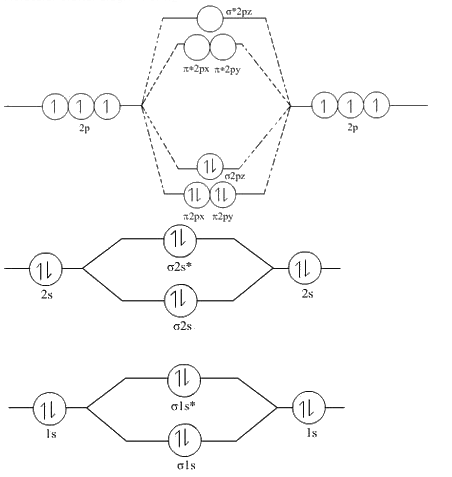
Draw molecular orbital diagram of n2 and calculate bond order
How are the quantam numbers n, l and m arrived at? Explain the significance of these quantam numbers. Write the postulates of Bohr's model of hydrogen atom.
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule.
Draw molecular orbital diagram of n2 and calculate bond order
Draw the molecular orbital diagram of N 2 and calculate the bond order. Molecular orbital diagram of N 2. Hence, bond order of N 2 is 3. Also calculate their bond order? Byju's Answer. Open in App. Molecular orbital diagram: The molecular orbital diagram describes the chemical bonding in a molecule based on molecular orbital theory MOT and linear combination of atomic orbital LCAO. The molecular orbital diagram has molecular orbital energy level at centre and is surrounded by atomic orbital energy level. It shows electrons in both bonding and anti-bonding molecular orbital. Draw the molecular orbital diagram of dioxygen and calculate bond order. Draw molecular orbital diagram of O 2 or N 2 with magnetic behavior and bond order. Write their electronic configuration, find the bond order and predict their magnetic behaviour.
Also calculate their bond order?
Formation of Nitrogen molecule by Molecular Orbital Theory:. On calculating bond order we ignore the combination of inner shells i. KK' as they have two electrons in both bonding and anti bonding orbitals. Nitrogen molecule has 3 bonds. Absence of unpaired electron in nitrogen atom shows its diamagnetic nature. Byju's Answer.
Understanding the bond order of a molecule is a crucial step in analyzing its chemical properties and reactivity. The bond order, derived from a molecular orbital diagram, provides insights into the strength, stability, and nature of chemical bonds within a molecule. What are Molecular Orbitals — Definition, Features 2. Molecular orbitals are regions of space where electrons are likely to be found in a molecule. They are formed by the overlapping of atomic orbitals , which are regions of space where electrons are localized around individual atoms. Molecular orbitals result from the linear combination of atomic orbitals, where atomic orbitals from different atoms overlap and create bonding and antibonding orbitals. Bonding orbitals are lower in energy and stabilize the molecule, while antibonding orbitals are higher in energy and destabilize the molecule. The bond order can be determined by comparing the number of electrons in bonding and antibonding orbitals.
Draw molecular orbital diagram of n2 and calculate bond order
None of the approaches we have described so far can adequately explain why some compounds are colored and others are not, why some substances with unpaired electrons are stable, and why others are effective semiconductors. These approaches also cannot describe the nature of resonance. Such limitations led to the development of a new approach to bonding in which electrons are not viewed as being localized between the nuclei of bonded atoms but are instead delocalized throughout the entire molecule. Just as with the valence bond theory, the approach we are about to discuss is based on a quantum mechanical model. Previously, we described the electrons in isolated atoms as having certain spatial distributions, called orbitals , each with a particular orbital energy. Just as the positions and energies of electrons in atoms can be described in terms of atomic orbitals AOs , the positions and energies of electrons in molecules can be described in terms of molecular orbitals MOs A particular spatial distribution of electrons in a molecule that is associated with a particular orbital energy. As the name suggests, molecular orbitals are not localized on a single atom but extend over the entire molecule. Consequently, the molecular orbital approach, called molecular orbital theory is a delocalized approach to bonding. Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms.
Cordova movies
Each sulfur atom contributes 6 valence electrons, for a total of 12 valence electrons. Although these two pairs are equivalent in energy, the np x orbital on one atom can interact with only the np x orbital on the other, and the np y orbital on one atom can interact with only the np y on the other. We might therefore expect it to have similar reactivity as alkali metals such as Li and Na with their single valence electrons. In addition, they are farther away from the nucleus than they were in the parent hydrogen 1 s atomic orbitals. Thus the interaction of filled shells always gives a bond order of 0, so filled shells are not a factor when predicting the stability of a species. Nonbonding Molecular Orbitals Molecular orbital theory is also able to explain the presence of lone pairs of electrons. Molecular Orbitals Involving Only ns Atomic Orbitals We begin our discussion of molecular orbitals with the simplest molecule, H 2 , formed from two isolated hydrogen atoms, each with a 1 s 1 electron configuration. Hence the electron density of bonding electrons is likely to be closer to the more electronegative atom. This ion has a total of three valence electrons. Molecular Orbital Theory. Experiments show that the He 2 molecule is actually less stable than two isolated He atoms due to unfavorable electron—electron and nucleus—nucleus interactions. Molecular orbital diagram: The molecular orbital diagram describes the chemical bonding in a molecule based on molecular orbital theory MOT and linear combination of atomic orbital LCAO. Electrons in nonbonding molecular orbitals have no effect on bond order. Molecular Orbitals Formed from ns and np Atomic Orbitals Atomic orbitals other than ns orbitals can also interact to form molecular orbitals.
For almost every covalent molecule that exists, we can now draw the Lewis structure, predict the electron-pair geometry, predict the molecular geometry, and come close to predicting bond angles. This electronic structure adheres to all the rules governing Lewis theory.
Of the three p orbitals, only one, designated as 3 p z , can interact with the H 1 s orbital. Conversely, the antibonding molecular orbitals are higher in energy, as shown. The molecular orbitals are no longer symmetrical, and the energies of the bonding molecular orbitals are more similar to those of the atomic orbitals of B. The magnetic properties of O 2 are not just a laboratory curiosity; they are absolutely crucial to the existence of life. The number of molecular orbitals is always equal to the total number of atomic orbitals we started with. Nonbonding Molecular Orbitals Molecular orbital theory is also able to explain the presence of lone pairs of electrons. Standard XII Chemistry. In contrast, most substances have only paired electrons. The number of electrons in an orbital is indicated by a superscript. Draw the molecular orbital energy-level diagram for the system. Consequently, electrons in such molecular orbitals are primarily located outside the internuclear region, leading to increased repulsions between the positively charged nuclei. Atomic orbitals other than ns orbitals can also interact to form molecular orbitals.

Something any more on that theme has incurred me.