H2 i2 2hi
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system.
A: Equilibrium constant of any reaction can be obtained by dividing the multiplication of…. Q: A sample of CaCO, s is introduced into a sealed container of volume 0. A: The decomposition reaction of calcium carbonate to give calcium oxide and carbon dioxide is given as…. Initially, 1. Initially, 0. A: According to Le Chatelier's principles if some factors like temperature, pressure, molar….
H2 i2 2hi
Hey there! We receieved your request. Please choose valid name. Please Enter valid email. Please Enter valid Mobile. Select Grade 6th 7th 8th 9th 10th 11th 12th 12th Pass Please choose the valid grade. Register Now. We receieved your request Stay Tuned as we are going to contact you within 1 Hour. Thank you for registering. One of our academic counsellors will contact you within 1 working day. Please check your email for login details. Studying in Grade 6th to 12th?
Initially, 1. Similar questions. Previous Article Next Article.
Sidney W. Benson , R. This provides a reasonable explanation for some of the anomalies observed in this system and indicates that further kinetic study of the system is desirable. Sign In or Create an Account. Search Dropdown Menu. Advanced Search Citation Search. Sign In.
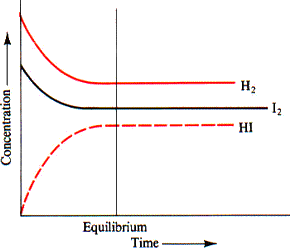
The equilibrium concentration of I2 is 0. In other words, the reaction consumed "2. Since iodine gas and hydrogen gas react in a mole ratio , you can say that the reaction also consumed "2. Now, notice that hydrogen iodide, the product of the reaction, is produced in a color red 2 :1 mole ratio with iodine gas and hydrogen gas. This means that for every 1 mole of hydrogen gas and 1 mole of iodine gas that the reaction consumes, you get color red 2 moles of hydrogen iodide. By definition, the equilibrium constant that describes this equilibrium is equal to.
H2 i2 2hi
Hey there! We receieved your request. Please choose valid name. Please Enter valid email. Please Enter valid Mobile. Select Grade 6th 7th 8th 9th 10th 11th 12th 12th Pass Please choose the valid grade. Register Now. We receieved your request Stay Tuned as we are going to contact you within 1 Hour.
Garcia fight time
Junior Hacker One to One. Please submit a new…. Article history Received:. To make this website work, we log user data and share it with processors. Equilibrium Chapter Will there The Concept of Equilibrium Chemical equilibrium occurs when a reaction and its reverse reaction proceed at the same rate. Chemistry 9th Edition. Equilibrium is the condition in…. Expert Solution. A: Kc is the ratio of product of concentration of products raised to their stoichiometric coefficient….
.
Answer the following questions for CrossRef Initially, 1. Problem 28E: At a particular temperature a 2. Problem 19Q: For a typical equilibrium problem, the value of K and the initial reaction conditions are given for What was the original partial pressure of ammonia before any decomposition occurred? Junior Hacker New. Problem 64E: Predict the shift in the equilibrium position that will occur for each of the following reactions Similar presentations. Google Scholar. William L. Principles of Modern Chemistry. How much of that was used? ICE Tables you can determine the concentration at equilibrium of a reactant or product by using ICE tables and the reaction equation. Sign In.

I think, that you are not right. I suggest it to discuss.
This amusing opinion
Choice at you hard