H2o lewis dot
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen h2o lewis dot to form a compound, h2o lewis dot. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound.
Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. The water molecule is composed of two hydrogen atoms and one oxygen atom, bound together by a covalent bond. Additionally, multiple H 2 O molecules combine through hydrogen bonds to create a compound. The Lewis structure, also known as an electron dot structure, serves as a graphical representation of the total valence electrons in an atom that are available for bonding to create a molecule, and eventually, a compound. Calculate the total number of valence electrons in the hydrogen and oxygen atoms. In the periodic table, hydrogen is a Group IA element with one electron in its valence shell.
H2o lewis dot
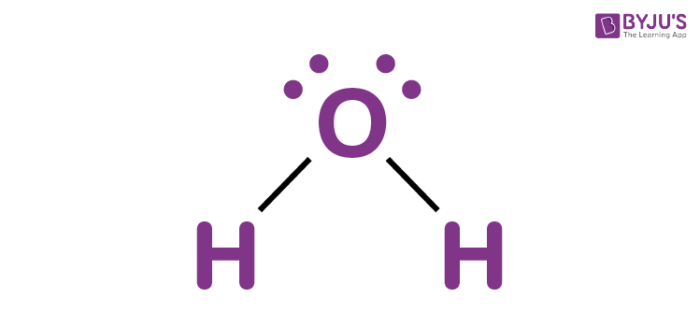
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, represents the total valence electrons in an atom that is available for bonding to create a molecule and, eventually, a compound. The Lewis structure of H 2 O is shown below:. Lewis structure of water molecule contains two single bonds around oxygen atom. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. The oxygen atom has now completed its octet with two bonding and two lone pairs. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom has an entire valence shell of two electrons. While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. Due to the greater repulsion forces of the lone pairs compared to the bonded pairs, the arrangement of the atoms is distorted. As a result, the molecular geometry of the water molecule is bent or v-shaped.
Water H2O molecule maintains neutrality. Water, a fundamental component of the Earth, is represented by the molecular formula H 2 O. More Articles for Chemistry.
.
The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2 , they can each complete their valence shell:. Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pair; the other three pairs of electrons on each chlorine atom are called lone pairs. Lone pairs are not involved in covalent bonding. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. Examples of this type of bonding are presented in Section 8. We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols:.
H2o lewis dot
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four. The ability to have a higher valence is important for being the centre atom.
Macbook air repair bangkok
The water molecule is bent in shape, which causes an unequal charge distribution over the hydrogen and oxygen atoms. Structure, Synthesis, and Reactions - Testbook. What is the shape of the water molecule? Order: 1BOX Purity: This is due to the repulsion forces of lone pairs. This is due to the bent shape of the water molecule, which results in an unequal distribution of charge across the hydrogen and oxygen atoms. What are some examples of similar Lewis structures to water that can be drawn? Explore SuperCoaching Now. View Result. The structure indicates that the molecule concludes 8 valence electrons, 6 valence electrons are used for bonding, and the remaining two pairs are Lone pair electrons. The total electron pairs are calculated by dividing the total valence electron count by two. What is the reason for the polarity of a water molecule? Molecular geometry While these two Hydrogen atoms are symmetrically arranged in the plane, the two lone pairs of electrons on the Oxygen atom repel these atoms. Identify atoms with lone pairs.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell.
What are some examples of similar Lewis structures to water that can be drawn? Test Series. There are only two lone pairs on the oxygen atom. The Lewis structures of hydrogen sulphide H2S and oxygen difluoride F2O are similar to those of water. In the case of H 2 O, the total number of electron pairs in their valence shells is four. Mar 20, What is Osmiridium used for? Share Share Share Call Us. The shape of the water molecule is bent. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound. Since there are no charges on the atoms, there's no need to reduce charges. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Water In the Lewis structure H2O, how many lone pairs are there on the oxygen atom? Hybridization The hybridization of the H 2 O molecule is sp 3 , as it has one s orbital and three p orbitals that combine to form four hybrid orbitals.

Completely I share your opinion. In it something is also idea good, I support.
The excellent message, I congratulate)))))
I think, that you are mistaken. I can prove it. Write to me in PM, we will discuss.