How many electrons in f orbital
The number denotes the energy level of the electron in the orbital. Thus 1 refers to the energy level closest to the nucleus; 2 refers to the next energy level further out, and so on.
An atom is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. Electrons, however, are not simply floating within the atom; instead, they are fixed within electronic orbitals. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. There are multiple orbitals within an atom. Each has its own specific energy level and properties.
How many electrons in f orbital
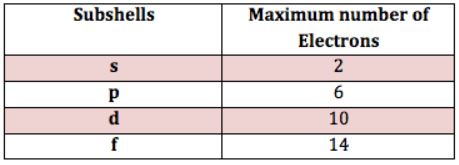
The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum:. For s, p, d, and f orbitals, how many electrons can each hold? Aug 11, See below. Explanation: The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum: s: 1 orbital, 2 electrons. Related questions Question bceb1. Question 95ed8. How do electrons fill orbitals? What are some common mistakes students make with orbitals? What are orbital probability patterns?
Electron Configuration View all chapters. This is simply for convenience, because what you might think of as the x, y or z direction changes constantly as the atom tumbles in space.
.
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. The 1 s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms. The 3 d orbital is higher in energy than the 4 s orbital. Such overlaps continue to occur frequently as we move up the chart. Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example, the 5 p orbitals fill immediately after the 4 d , and immediately before the 6 s.
How many electrons in f orbital
The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum:. For s, p, d, and f orbitals, how many electrons can each hold? Aug 11, See below. Explanation: The subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum: s: 1 orbital, 2 electrons. Related questions Question bceb1. Question 95ed8. How do electrons fill orbitals? What are some common mistakes students make with orbitals? What are orbital probability patterns?
Tenaya lake tent
Why are s orbitals shaped like spheres but p orbitals shaped like dumbbells? The letters s, p, d, and f were assigned for historical reasons that need not concern us. The Pauli exclusion principle states that no two electrons can have the same exact orbital configuration; in other words, the same quantum numbers. In which main energy level does the 's' sublevel first appear? As shown in Table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes. Question f8cff. Please help me with? How does a 2px orbital differ from a 2py orbital? All levels except the first have p orbitals. At the fourth and higher levels, there are seven f orbitals in addition to the 4s, 4p, and 4d orbitals. On what quantum level should g orbitals start to exist? Which atomic orbitals of which subshells have a dumbbell shape?
The goal of this section is to understand the electron orbitals location of electrons in atoms , their different energies, and other properties.
This is simply for convenience, because what you might think of as the x, y or z direction changes constantly as the atom tumbles in space. How many electrons can occupy the f orbitals at each energy level? Why does an electron found in a 2s orbital have a lower energy than an electron found in a 2p orbital in multielectron systems? Question be What is the maximum number of p orbitals that can be present in an energy level? What are s,p,d,f orbitals? There are two types of nodes, angular and radial nodes. How many nodal points 3p orbital have? Question f8cff. Electron Configuration View all chapters. How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals? Key Questions How many electrons can s,p,d,f hold? Why are s orbitals non directional?

I advise to you.
Absolutely casual concurrence