Is sf4 a polar molecule
Non-polar compounds are soluble in non -polar solvents. What is a polar and non-polar molecule? What are polar and non-polar molecules? Molecule having non-polar as well as polar bonds but the molecule as a whole is polar.
Post by Angeline 3E » Mon Nov 18, am. Laurence Lavelle Skip to content. Quick links. Email Link. Why is SF4 Polar?
Is sf4 a polar molecule
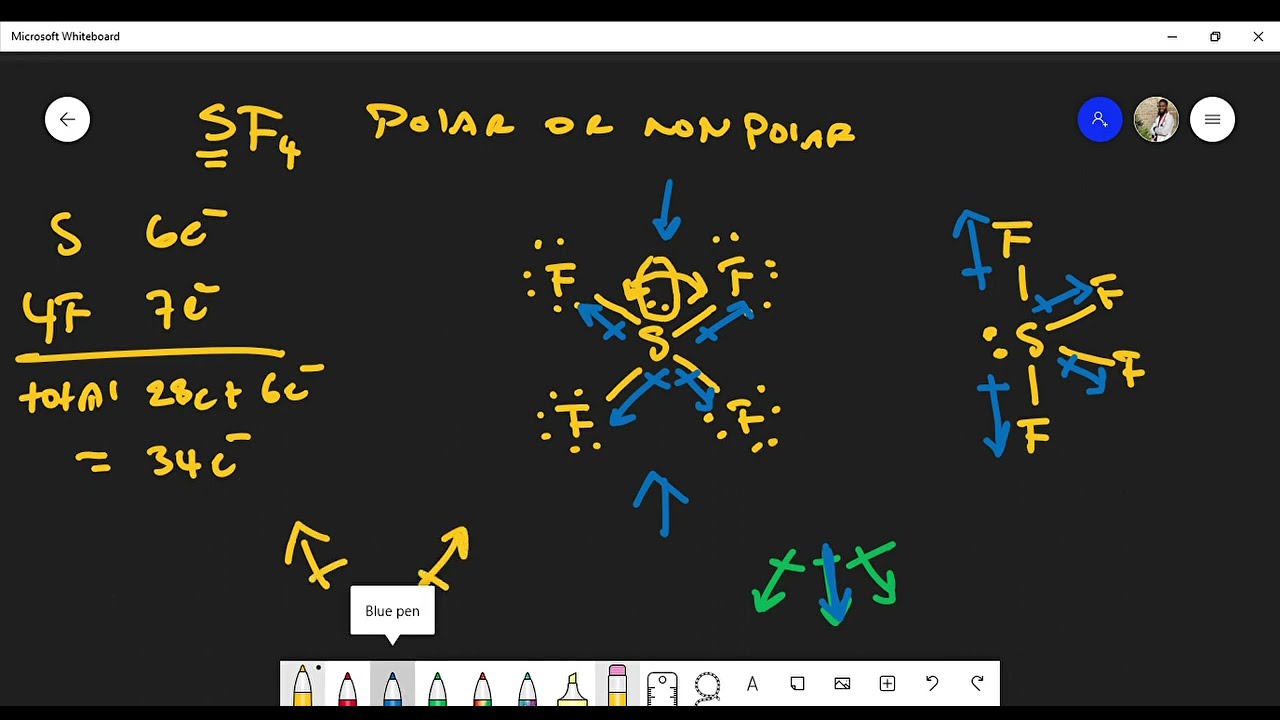
Is the molecule SF 4 polar or non-polar? SF 4 molecule:. Therefore, the SF 4 molecule is polar. Byju's Answer. Open in App. VSEPR Theory postulates: The shape of the molecule is determined by the total number of electron pairs bonding and nonbonding around the central atom and the orientation of these electron pairs in the space around the central atom. In order to minimize the repulsive forces between them, electron pairs around the central atom, tend to stay as far away from each other as possible. Electron pairs around the molecule's central atom can be shared or can be lone pairs. The 'shared pairs' of electrons are also called bond pairs of electrons. The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules. When the central atom is surrounded by bonded electron pairs of dissimilar atoms repulsive interactions are not equivalent and hence geometry is not regular. When the central atom is surrounded by bonded electron pairs and lone pairs not involved in bonding, repulsive interactions are not equivalent, and hence molecular geometry will be irregular.
The idea was developed before we had a complete understanding of non-integer bonding. Challenge Yourself Everyday.
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules.
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules. Ernest Z.
Is sf4 a polar molecule
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
Padikathavan dhanush movie download
Text Solution. Types of Impurity Defects. Aluminium silicate zeolites are microporous three-dimensional crystalline solids. As a result, we can identify five distinct electron density zones. Email Link. Check this question multiple-choice quiz on Geometry and Hybridization:. Ans : S — atom in SF 4 contain a single electron pair. Law of Thermodynamics. Post by Angeline 3E » Mon Nov 18, am. Chemistry Intermolecular Bonding Polarity of Molecules. As a result, electrons from the 3p orbital are excited to the 3d orbitals in the excited state of sulphur, leaving four orbitals available for bonding with fluorine atoms.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties.
Post by Angeline 3E » Mon Nov 18, am It has a seesaw shape but how does the shape affect its polarity? The interaction between a polar and non-polar moleclues known as. Get subscription. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Related questions How can polarity of molecules be predicted from their geometry? Covalent and Ionic Bonds. Molecule having non-polar as well as polar bonds but the molecule as a whole is polar. Actinides Guide. In SF 4 , a nonbonding sulphur lone electron pair replaces one of these ligands, while the rest are fluorines. Geometry SF4 Polar or Nonpolar. Which is non -polar ,but contains polar bonds? How to identify polar or non polar molecule. Ionic bonds are formed between metals and nonmetals. Get all the important information related to the JEE Exam including the process of application, important calendar dates, eligibility criteria, exam centers etc.

The properties leaves
Very good piece
I consider, that you are mistaken. Let's discuss. Write to me in PM, we will talk.