Lewis dot diagram for h2o
Water, one of the Earth's primary constituents, has the molecular formula H 2 O. A water molecule comprises two hydrogen atoms and one oxygen atom joined by a covalent bond. Furthermore, two or more H 2 O molecules join by hydrogen bonds to form a compound.
When studying chemical species, understanding the arrangement of their valence electrons is key. Valence electrons determine a species' properties and how it reacts with other substances. However, drawing out all of the electron shells can be complex and time-consuming, especially for larger molecules. Enter the Lewis dot diagram. A Lewis dot diagram is a simplified representation of a molecule's valence electrons. It shows the arrangement of atoms, valence electrons, and their bonding. In this article, we'll explore Lewis dot diagrams in chemistry.
Lewis dot diagram for h2o
.
Dynamic Equilibrium.
.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell. For representative elements, the number of valence electrons equals the group number on the periodic table. To draw the Lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron — never seven. Also, helium is shown in group 8A, but it only has two valence electrons. Nonmetals can form a chemical bond by sharing two electrons.
Lewis dot diagram for h2o
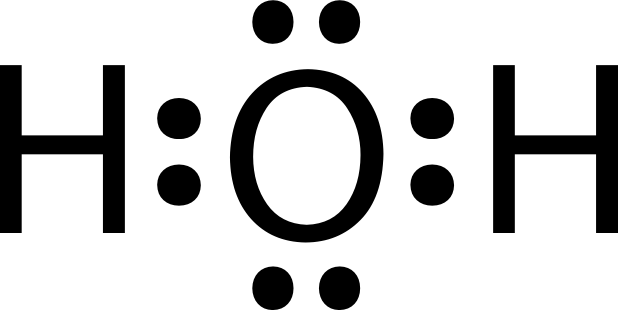
A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound. The Lewis structure, also known as an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound. Determine the total number of electrons in the valence shells of hydrogen and oxygen atoms. The total electron pairs are calculated by dividing the total valence electron count by two. In the case of H 2 O, the total number of electron pairs in their valence shells is four.
Final fantasy tactics advance gamefaqs
Water, the essential substance for life as we know it, is a compound with the chemical formula H2O. Aldehydes and Ketones. Gas Chromatography. Physical Chemistry. Factors Affecting Reaction Rates. Condensation Polymers. The oxygen atoms have a full outer shell of electrons, but the central carbon atom only has four electrons in its outer shell. We need to form additional covalent bonds to fill its outer shell. Understanding Lewis Dot Diagrams: Examples and Key Features In the previous section, we introduced the concept of a Lewis dot diagram as a simplified way of representing a molecule's valence electrons. Metallic Solids. Reactions of Halogens. It shows the arrangement of atoms, valence electrons, and their bonding. Reactions of Halides.
Lewis structure of water molecule contains two single bonds around oxygen atom.
We'll start by explaining what they are before delving into some common examples. Period 3 Elements. Count up the total number of electrons that you've already added. Mar 13, Electrode Potential. Acid-Base Reactions. Mass Spectrometry. This gives the carbon atom a total of eight electrons in its outer shell, completing its octet. Plus, drawing Lewis dot diagrams is a great way to simplify complex chemical structures and make them easier to understand. With practice, drawing and interpreting Lewis dot diagrams will become second nature, and you'll be well on your way to mastering the fundamentals of chemistry.

In my opinion you commit an error. Let's discuss.