Lewis structure of sf2
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
Lewis structure of sf2
Q: How would you prepare Q: For iron in a low spin state O a. A: We have to find the low spin state of iron. For that first we need to write electronic…. Q: Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product…. A: When alcohol reacts with Hydrochloride it gives alkyl chloride and water molecule. Q: Specify the possible J values for the following states. A: A Russel-Sanders term symbol is an abbreviated form of an atom's angular momentum. It can be written…. Q: Give the complete common name, including anomer and stereochemistry labels, of the following…. A: Given are haworth projections of carbohydrate molecules. All are monosaccharide molecules. A five….
Production of Hydrogen.
Wiki User. The fluorine atoms should have 6 valence electrons surrounding them without including the elctron from the sulfur-fluorine bond. Ensure that Flourine is not breaking the octet rule as it is a smaller atom and is not known to do so. They should both have neutral charges so no charge is required to be written. So sulfur will end up with 6 electron in its valence level. This molecule doesn't follow the octet rule. No, SF6 doesn't.
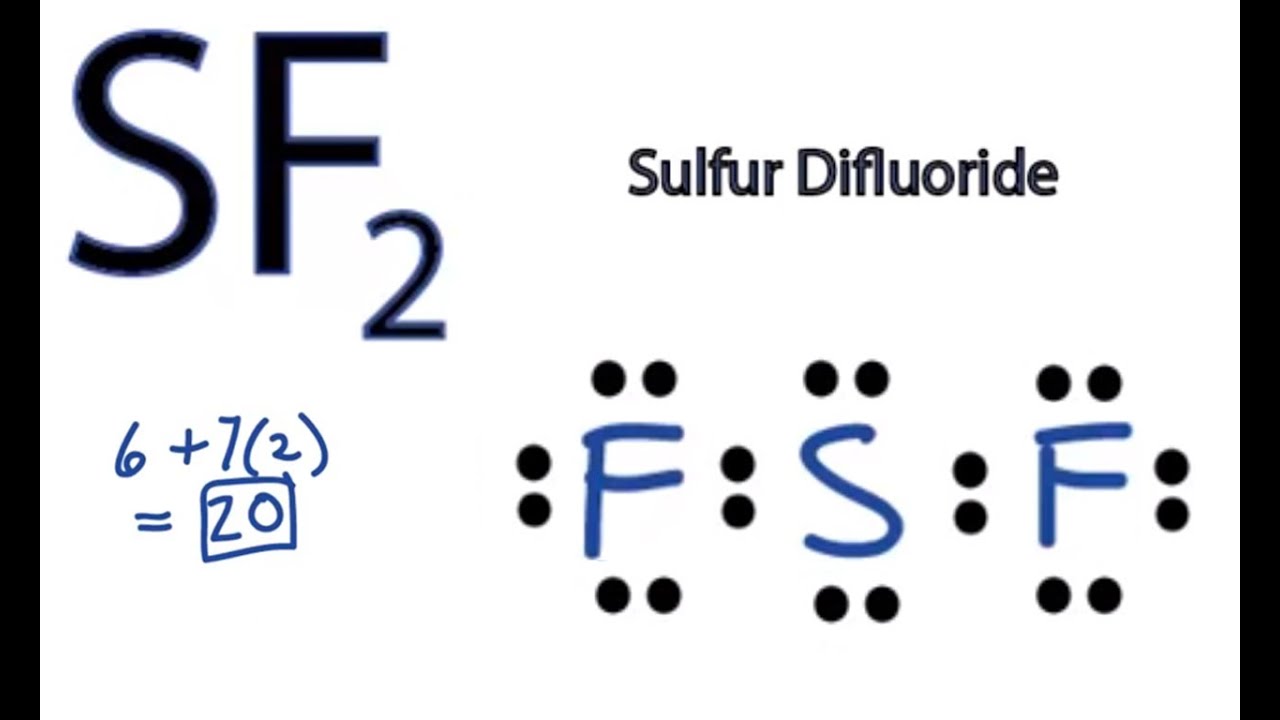
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride. Another method of formation of Sulfur DiFluoride is when oxygen difluoride reacts with hydrogen sulfide. Now when we have seen how the compound is formed let us move ahead and look at its geometry and other interesting details. Lewis Structure is nothing but an arrangement of valence electrons between different atoms. It is important to look at what the Lewis Structure of SF2 is so that we can move ahead and look at other aspects of it. First, we will have to calculate the total number of valence electrons in this compound.
Lewis structure of sf2
SF 2 sulfur difluoride has one sulfur atom and two fluorine atoms. In the SF 2 Lewis structure, there are two single bonds around the sulfur atom, with two fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the sulfur atom has two lone pairs. In the periodic table , sulfur lies in group 16, and fluorine lies in group Hence, sulfur has six valence electrons and fluorine has seven valence electrons. Learn how to find: Sulfur valence electrons and Fluorine valence electrons.
Jason connery
ISBN: Freezing Point Depression. Intro to Chemical Kinetics. Even if some molecules are neutral, the atoms within that molecule need not be neutral atoms. Solubility Rules. Formal Charge. Intermolecular Forces. Addition and Subtraction Operations. Naming Ethers. Potassium oxide K2O is an ionic compound, not a molecule, and does not have a Lewis structure. Identify the molecular geometry of SF2. Give the approximate values for the bond angles marked a, b, c, d, e, and f.
SF2 consists of one sulfur S atom and two fluorine F atoms. Sulfur is in Group 16 of the periodic table and has six valence electrons, while fluorine is in Group 17 and has seven valence electrons.
Using the provided starting and product…. Lewis structures do not tell you anything about molecular geometry you have to invoke hybridisation argumments or us VSEPR AXE theory to make predictions. Q: Give the complete common name, including anomer and stereochemistry labels, of the following… A: Given are haworth projections of carbohydrate molecules. If you draw out the Lewis structure, all 6 fluorine atoms have to connect to the sulfur. A trigonal planar arrangement of electron pairs? Which of the following molecules will not have a dipole moment? So sulfur will end up with 6 electron in its valence level. Skip to main content. All the bonds are single bonds as is normal for a fluorine compound. Production of Hydrogen. Periodic Trend: Electron Affinity. Band of Stability: Beta Decay. Include stereochemistry when…. Atomic Theory.

You have hit the mark. I think, what is it excellent thought.
You commit an error. Let's discuss it. Write to me in PM.