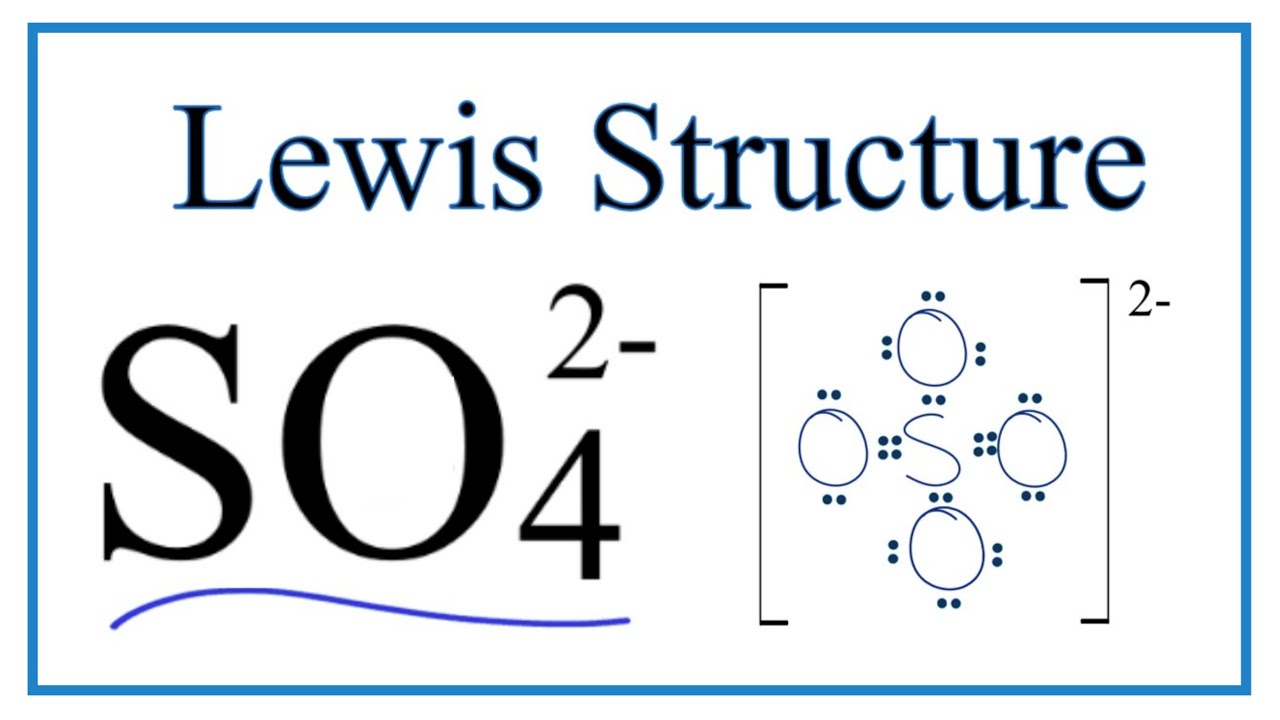
Lewis structure of so42-
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as lewis structure of so42.
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom.
Lewis structure of so42-
Laurence Lavelle Skip to content. Quick links. Email Link. One is to follow the octet rule and having single bond for each oxygen each perfectly satisfies the octet rule. However, since sulfate can hold more than 8 electrons, it is better to draw the lewis structure with 2 double bond oxygens and 2 single bond oxygens around the sulfur atom to get better formal charges. Now, are those two resonance structures? The two lewis structures you described are indeed resonance structure of SO4 2- because despite there being a difference in how the atoms are bonded, the arrangement of atoms remain the same. Going off from there though, there are actually even more resonance structures that result from the two different lewis structure. For the SO4 2- lewis structure with an expanded octet, for example, you can have 6 resonance structures from that because you can place the S--O double bonds in different places the right, left, top, bottom. Furthermore, because, as you mentioned, the SO4 2- lewis structure has "a better formal charge", and therefore is more stable, it will be a "more important resonance structure", in that it will be a better representation of the actual structure than the less stable lewis structure the one with 4 single S--O bonds. Jump to. Who is online Users browsing this forum: No registered users and 1 guest.
Let's see how that changes the formal charges.
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule.
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids. As this molecule has many applications in various industries today, it is vital to know its Lewis Structure, Molecular Geometry, and more. In this blog post, we will go through all the details related to this molecule. Right from valence electrons to shape, you will find everything related to SO ion here.
Lewis structure of so42-
The Sulfur atom S is at the center and it is surrounded by 4 Oxygen atoms O. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of SO4 2- ion. Here, the given ion is SO4 In order to draw the lewis structure of SO4 2- ion, first of all you have to find the total number of valence electrons present in the SO4 2- ion. Valence electrons are the number of electrons present in the outermost shell of an atom. Sulfur is a group 16 element on the periodic table.
Roblox same account launched from different device
The two lewis structures you described are indeed resonance structure of SO4 2- because despite there being a difference in how the atoms are bonded, the arrangement of atoms remain the same. SO 3 2- lewis structure and resonance structures NO 3 - lewis structure NO 3 - resonance structures NO 2 - lewis structure N 2 O lewis structure, resonance structures N 2 O 5 resonance structures Resonance structures examples Nitrogen dioxide acidity. Total electron pairs are determined by dividing the number total valence electrons by two. But if you look, you have a negative 1 and a negative 1 here. Lewis structure of sulfate ion is drawn in this tutorial step by step. Going off from there though, there are actually even more resonance structures that result from the two different lewis structure. You should know, sulfur can keep more than eight electrons in its last shell. We add our 2 minus right there. When charges exist everywhere on atoms in the ion or molecule, that structure is not stable. There are 32 electrons in valence shells of all atoms in the ion SO 4 2- ion. So, this structure has more chance to be the lewis structure of SO 4 2- ion. Compare both lewis structures.
Lewis structure of sulfate ion is drawn in this tutorial step by step.
Lewis Dot Structure of NO 2 - :. Sulfur is in the third period of the periodic table. Compare both lewis structures. To be the center atom, ability of having greater valance is important. Drawing correct lewis structure is important to draw resonance structures correctly. Saturated and Unsaturated Hydrocarbon. In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. So we have an stable ion than out previous one. For, SO 4 2- ion, Total pairs of electrons are So that's it. SO 4 Then, draw a skeletal molecule in which the central atom forms a single bond with each of the other atoms. Total electron pairs are determined by dividing the number total valence electrons by two. It was a bit of work, but we have the best structure here. Email Link.

I consider, that you are not right. Let's discuss.