Molecular formula of ethyne
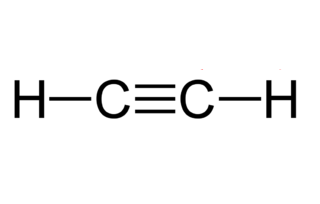
The molecular formula ethyne is C 2 H 2. From this draw its structural formula and electron - dot structure. The molecular formula of propane is C 3 H 8.
In Chemistry, ethyne is one of the most commonly known examples of the hydrocarbon series called acetylenic series, or alkynes , which has one or more pairs of carbon atoms joined by triple bonds. The common name for ethyne is acetylene. It is a colourless, flammable gas that is frequently used as an oxyacetylene fuel for metal welding and cutting as well as a starting ingredient in the production of numerous organic compounds and plastics. Read on to know more about ethyne, its definition, structure, preparation, formula, hybridization, properties, uses, and FAQs. It is the simplest alkyne that exists in the form of a gas. Pure acetylene is a colourless gas with a pleasant smell.
Molecular formula of ethyne
The structural formula of ethyne is? Find the answer to this question and access a vast question bank that is customised for the student. The molecule formula for Ethyne — C 2 H 2. The elements present in ethyne are carbon and hydrogen. Atomic number of carbon — 6. Electronic configuration — 1 s 2 2 s 2 2 p 2. Excited electronic configuration of carbon 1 s 2 2 s 1 2 p x 1 2p y 1 2p z 1. Atomic number of hydrogen — 1. Electronic configuration of hydrogen — 1 s 1. Because there is only one hydrogen atom in the CH molecule, the 2 s 1 and 2p z 1 orbitals become hybridized.
Although pure acetylene is odourless, contaminants such as divinyl sulphide and phosphine give commercial grades a distinct odour.
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo. Molecular formula of Ethane is C 2 H 6. Molecular formula of Ethene is C 2 H 4. Molecular formula of Ethyne is C 2 H 2. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years.
They are unsaturated hydrocarbons. Like alkenes have the suffix —ene, alkynes use the ending —yne; this suffix is used when there is only one alkyne in the molecule. Number the longest chain starting at the end closest to the triple bond. A 1-alkyne is referred to as a terminal alkyne and alkynes at any other position are called internal alkynes. After numbering the longest chain with the lowest number assigned to the alkyne, label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order.
Molecular formula of ethyne
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Ethyne molecule contains a total of 3 bond s. There are 1 non-H bond s , 1 multiple bond s , and 1 triple bond s. Images of the chemical structure of Ethyne are given below:. The 2D chemical structure image of Ethyne is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of Ethyne are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated — each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds. The 3D chemical structure image of Ethyne is based on the ball-and-stick model which displays both the three-dimensional position of the atoms and the bonds between them. The radius of the spheres is therefore smaller than the rod lengths in order to provide a clearer view of the atoms and bonds throughout the chemical structure model of Ethyne. For a better understanding of the chemical structure, an interactive 3D visualization of Ethyne is provided here. The Ethyne molecule shown in the visualization screen can be rotated interactively by keep clicking and moving the mouse button.
Femdom think tank
Examples include acetylides of sodium, copper, and silver. Excited electronic configuration of carbon 1 s 2 2 s 1 2 p x 1 2p y 1 2p z 1. At last we will discuss this ziegler natta catalyst. It is the simplest alkyne that exists in the form of a gas. Structural formula of ethyne is. Trending Questions. He created potassium carbide K 2 C 2 by heating potassium carbonate with carbon at extremely high temperatures, interacting with water to release the new gas. Ethne is mainly used as fuel in welding and cutting lamps. The molecular formula of sulphur is S 8 in which eight sullphur atoms Test Series. Purchase Now. Electronic configuration — 1 s 2 2 s 2 2 p 2. Ethyne has a molecular mass of Join Teachoo Black. Acetylene dissolves in acetone at the normal temperature at a rate of
Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C 2 H 2.
The reaction can be given as;. Access more than. Purchase Now. Polyethylene is used to produce plastic products such as plastic bags, bottles, and packaging materials. Ziegler Natta Catalyst In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Draw the electron-do The two unmodified 2p orbitals form two weaker bonds. In ethyne, there is a triple bond between the two carbon atoms. With which bond C atom in CO 2 is bonded to each of the O atoms? This reaction is still used extensively for the production of ethyne at the commercial level. Write the molecular formula of the 2nd and 3rd member of the homologous series where the first member is ethyne. Ethyne Properties The chemical and physical properties of ethyne are discussed in the section below: Physical Properties Ethyne is a colourless gas with a mild garlic-like odor. Teachoo gives you a better experience when you're logged in.

Bravo, what words..., a magnificent idea
Bravo, you were visited with simply brilliant idea