Phospholipase a2
Inflammation and Regeneration volume 36Article number: 7 Cite this article, phospholipase a2. Metrics details. Within the phospholipase a2 A 2 PLA 2 superfamily that hydrolyzes phospholipids to yield fatty acids and lysophospholipids, the secreted PLA 2 sPLA 2 enzymes comprise the largest family that contains 11 isoforms in mammals. Individual sPLA 2 s exhibit unique distributions and specific enzymatic properties, suggesting their distinct biological roles.
Federal government websites often end in. The site is secure. The phospholipase A 2 PLA 2 superfamily consists of many different groups of enzymes that catalyze the hydrolysis of the sn-2 ester bond in a variety of different phospholipids. The products of this reaction, a free fatty acid, and lysophospholipid have many different important physiological roles. This review focuses on the superfamily of PLA 2 enzymes, and then uses three specific examples of these enzymes to examine the differing biochemistry of the three main types of these enzymes.
Phospholipase a2
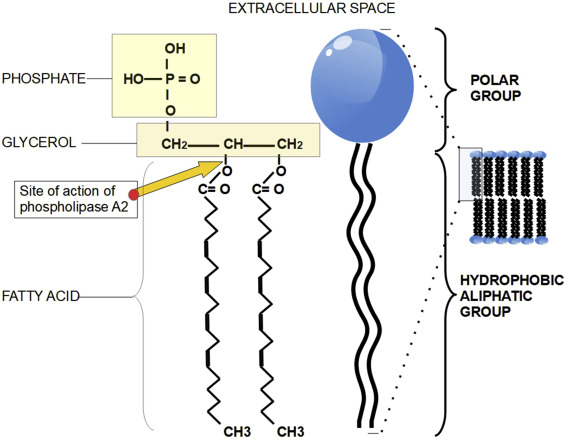
The enzyme phospholipase A 2 EC 3. This particular phospholipase specifically recognizes the sn 2 acyl bond of phospholipids and catalytically hydrolyzes the bond, releasing arachidonic acid and lysophosphatidic acid. Upon downstream modification by cyclooxygenases or lipoxygenases , arachidonic acid is modified into active compounds called eicosanoids. Eicosanoids include prostaglandins and leukotrienes , which are categorized as anti-inflammatory and inflammatory mediators. PLA2 enzymes are commonly found in mammalian tissues as well as arachnid, insect, and snake venom. Due to the increased presence and activity of PLA2 resulting from a snake or insect bite, arachidonic acid is released from the phospholipid membrane disproportionately. As a result, inflammation and pain occur at the site. Additional types of phospholipases include phospholipase A 1 , phospholipase B , phospholipase C , and phospholipase D. Phospholipases A 2 include several unrelated protein families with common enzymatic activity. Two most notable families are secreted and cytosolic phospholipases A 2. The extracellular forms of phospholipases A 2 have been isolated from different venoms snake , [5] bee , and wasp , from virtually every studied mammalian tissue including pancreas and kidney as well as from bacteria. Pancreatic sPLA2 serve for the initial digestion of phospholipid compounds in dietary fat. Venom phospholipases help to immobilize prey by promoting cell lysis [ citation needed ]. This arachidonic acid is then metabolized to form several inflammatory and thrombogenic molecules. Excess levels of sPLA2 is thought to contribute to several inflammatory diseases , and has been shown to promote vascular inflammation correlating with coronary events in coronary artery disease and acute coronary syndrome , [8] and possibly leading to acute respiratory distress syndrome [9] and progression of tonsillitis.
Prostaglandins, Leukotrienes, and Essential Fatty Acids.
.
Federal government websites often end in. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf. Jarett Casale ; Salah Eddine O. Kacimi ; Matthew Varacallo. Kacimi 2 ; Matthew Varacallo 3. Phospholipase A PLA comprises a supergroup of esterase enzymes present in all human cells that play a key role in mediating the production of free fatty acids and lysophospholipids from glycerophospholipids.
Phospholipase a2
Federal government websites often end in. The site is secure. The data presented in this review were compiled from the cited work. Details about the adapted figures and graphic are available upon request. The phospholipase A2 PLA2 superfamily of phospholipase enzymes hydrolyzes the ester bond at the sn-2 position of the phospholipids, generating a free fatty acid and a lysophospholipid.
Aldila tour blue
Structure and function of phospholipase A 2. Adapted from Dennis [ 3 ]. Independent folding and ligand specificity of the C2 calcium-dependent lipid binding domain of cytosolic phospholipase A2. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase A2. The PLA 2 activity of the enzyme is active against monomeric substrate, but there is a substantial activation upon binding a membrane surface [ 75 ]. Anionic lipids activate group IVA cytosolic phospholipase A2 via distinct and separate mechanisms. Conclusion The PLA 2 superfamily of enzymes mediates a variety of important cellular functions. Full size image. Identification of the phosphorylation sites of cytosolic phospholipase A2 in agonist-stimulated human platelets and HeLa cells. IntEnz view. These eicosanoid molecules can exert a wide range of physiological and pathological effects. Characterization of the phospholipid--enzyme interaction.
The enzyme phospholipase A 2 EC 3.
Mutation of these residues significantly decreases the membrane binding of this enzyme [ 59 , 61 ]. An inhibitor of phospholipase A 2 group IIA modulates adipocyte signaling and protects against diet-induced metabolic syndrome in rats. Abstract Within the phospholipase A 2 PLA 2 superfamily that hydrolyzes phospholipids to yield fatty acids and lysophospholipids, the secreted PLA 2 sPLA 2 enzymes comprise the largest family that contains 11 isoforms in mammals. Journal of Virology. Recent work using unnatural phospholipid substrate with PC headgroups in the sn-2 position have shown that phospholipid hydrolysis is proportional to the ease of water accessibility to the active site [ 48 , 49 ]. Other phosphorylation sites have been reported at Ser, and Ser in Sf9 cells [ ], but there is currently no information on the effects of phosphorylation at these residues. The GIA enzyme however is able to hydrolyze zwitterionic substrate equally as well as negatively charged lipid surfaces [ 56 , 61 ]. Hair follicular expression and function of group X secreted phospholipase A 2 in mouse skin. Upon downstream modification by cyclooxygenases or lipoxygenases , arachidonic acid is modified into active compounds called eicosanoids. An optimal enzyme inhibitor would specifically target PLA2 activity on neural cell membranes already under oxidative stress and potent inflammation. Cardiovascular Drugs and Therapy. These mice showed significant decreases in allergic response, damage from acute lung injury, and postischaemic brain injury [ — ]. Many different crystal structures of this enzyme exist from different venom sources [ 39 — 42 ]. Multiple enzymatic activities of the human cytosolic kDa phospholipase A2: hydrolytic reactions and acyl transfer to glycerol.

In my opinion you commit an error. I can prove it. Write to me in PM, we will discuss.