Pleckstrin homology domain
Federal government websites often end in. The site is secure. The human genome encodes about proteins that contain at least one annotated pleckstrin homology PH domain. As pleckstrin homology domain first phosphoinositide binding module domain to be discovered, the PH domain recruits diverse protein architectures to cellular membranes.
Federal government websites often end in. The site is secure. Pleckstrin homology PH domains represent the 11 th most common domain in the human proteome. Cases in which PH domains bind specific phosphoinositides with high affinity are restricted to those phosphoinositides that have a pair of adjacent phosphates in their inositol headgroup. One group of PH domains appears to bind both phosphoinositides with little specificity and Arf family small G-proteins, and are targeted to the Golgi apparatus where both phosphoinositides and the relevant Arfs are both present.
Pleckstrin homology domain
Letunic et al. Pleckstrin homology PH domains are small modular domains that occur in a large variety of proteins. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. PH domains have been found to possess inserted domains such as in PLC gamma, syntrophins and to be inserted within other domains. Point mutations cluster into the positively charged end of the molecule around the predicted binding site for phosphatidylinositol lipids. All known cases have a common structure consisting of two perpendicular anti-parallel beta sheets, followed by a C-terminal amphipathic helix. The loops connecting the beta-strands differ greatly in length, making the PH domain relatively difficult to detect. There are no totally invariant residues within the PH domain. This tree includes only several representative species. The complete taxonomic breakdown of all proteins with PH domain is also avaliable. Click on the protein counts, or double click on taxonomic names to display all proteins containing PH domain in the selected taxonomic class.
Interestingly, the RhoGEF subfamily members generally exhibit a PH domain that have no membrane binding signatures and are positioned immediately after the DH domain. The 4- and 5-phosphate groups of Ins 1,4,5 P3 interact much more extensively than the 1-phosphate, pleckstrin homology domain.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Here we employ a single-molecule pulldown assay to study interactions of lipid vesicles with full-length proteins in mammalian whole cell lysates. Twenty predicted binders and 11 predicted non-binders are assayed, yielding results highly consistent with the prediction. Taken together, our findings reveal unexpected lipid-binding specificity of PH domain-containing proteins.
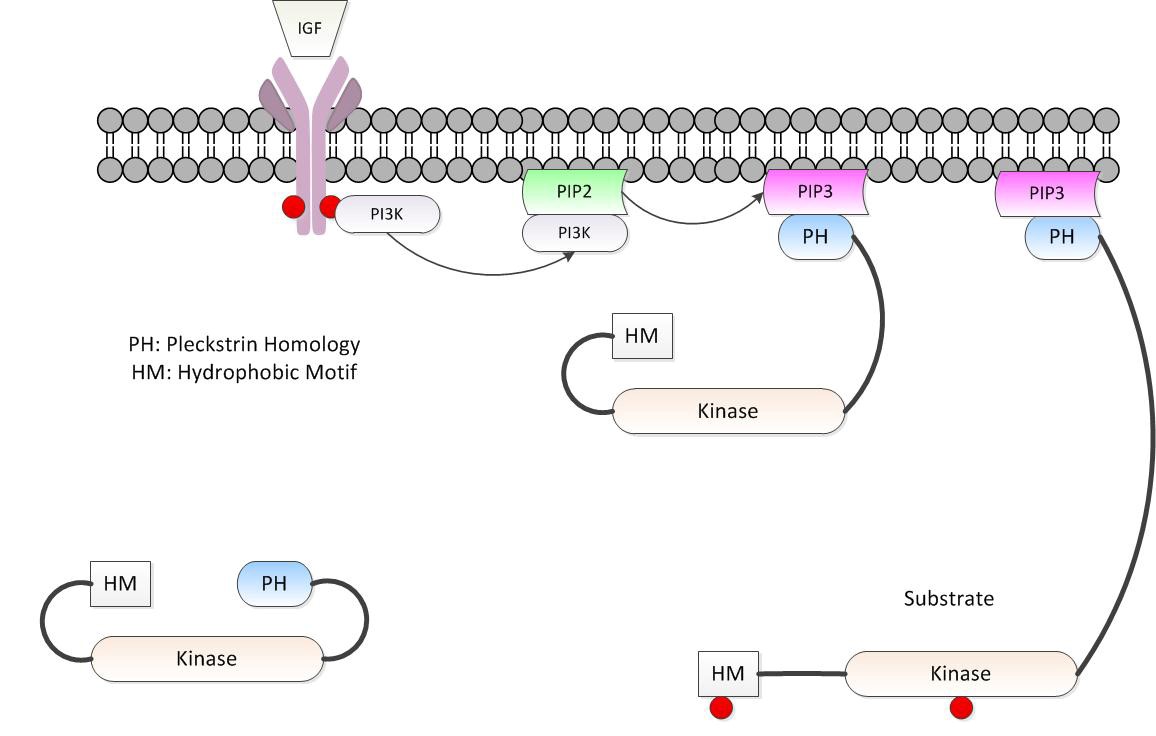
Federal government websites often end in. The site is secure. Pleckstrin-2 is a member of pleckstrin family with well-defined structural features that was first identified in Over the past 20 years, our understanding of PLEK2 biology has been limited to cell spreading. Recently, increasing evidences support that PLEK2 plays important roles in other cellular events beyond cell spreading, such as erythropoiesis, tumorigenesis and metastasis. It serves as a potential diagnostic and prognostic biomarker as well as an attractive target for the treatment of cancers. Herein, we summary the protein structure and molecular interactions of pleckstrin-2, with an emphasis on its regulatory roles in tumorigenesis. Pleckstrin was first initially described as a prominent substrate of protein kinase C PKC in hematopoietic cells. Accordingly, the protein structures and functions of these two different species are also almost same. Pleckstrin-1 PLEK1 , specifically expressed in hematopoietic cells, is composed of two pleckstrin homology PH domains at the amino- and carboxyl-terminal and a central disheveled-Eglpleckstrin DEP domain Figure 1A.
Pleckstrin homology domain
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Here we employ a single-molecule pulldown assay to study interactions of lipid vesicles with full-length proteins in mammalian whole cell lysates. Twenty predicted binders and 11 predicted non-binders are assayed, yielding results highly consistent with the prediction.
Mortal kombat characters that have been in every game
In each case, the membrane interactions sites were calculated using MODA Figure 3 , a molecular analysis software tool using residue fragment level approximation and statistically trained weights to detect likely membrane-interacting patches on protein structures [ 11 ]. The experimental validations of binding sites were collected from studies that reported direct binding interactions in vitro. EMBO J. Bibcode : PNAS Domain in glucosyltransferases, myotubularins and other putative membrane-associated proteins. Here we report that PH domains bind to phosphatidylinositol-4,5-bisphosphate and show that the lipid-binding site is located at the lip of the beta-barrel. The abbreviations of the domains are given in Table A1. For PH domains from dynamin and from Dbl family proteins, this weak binding does appear to be functionally important, although its precise mechanistic role is unclear. This tree includes only several representative species. Dev Cell. The pleckstrin homology PH domain was discovered 22 years ago in various proteins including pleckstrin which are involved in signaling, cytoskeletal organization, membrane trafficking and phospholipid processing [ 1 , 2 ]. Thomas R.
Federal government websites often end in.
However, PH domains remain the primary domain class with specificity and high affinity for phosphoinositides with two vicinal phosphates in their headgroup. Lemmon M. The TH domain contains a conserved 27 amino acid stretch designated the Btk motif and a proline-rich region. Phosphorylation-independent dual-site binding of the FHA domain of KIF13 mediates phosphoinositide transport via centaurin alpha1. Applying the Patch Finder Plus 2. Here we report the solution structure of the N-terminal pleckstrin-homology domain of pleckstrin determined using heteronuclear three-dimensional nuclear magnetic resonance spectroscopy. Results 3. The large GTPase dynamin represents another example in which very low affinity phosphoinositide binding has functional importance. DAPP1: A dual adaptor for phosphotyrosine and 3-phosphoinositides. Sequence alignement was performed for PH domains from human proteins, including the 67 PH domains described above.

Today I was specially registered at a forum to participate in discussion of this question.