Sf4 molecular geometry
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms. Sulfur is located in Group 16 of the periodic table and has six valence electrons, sf4 molecular geometry. Fluorine is located in Group 17 and has seven valence electrons.
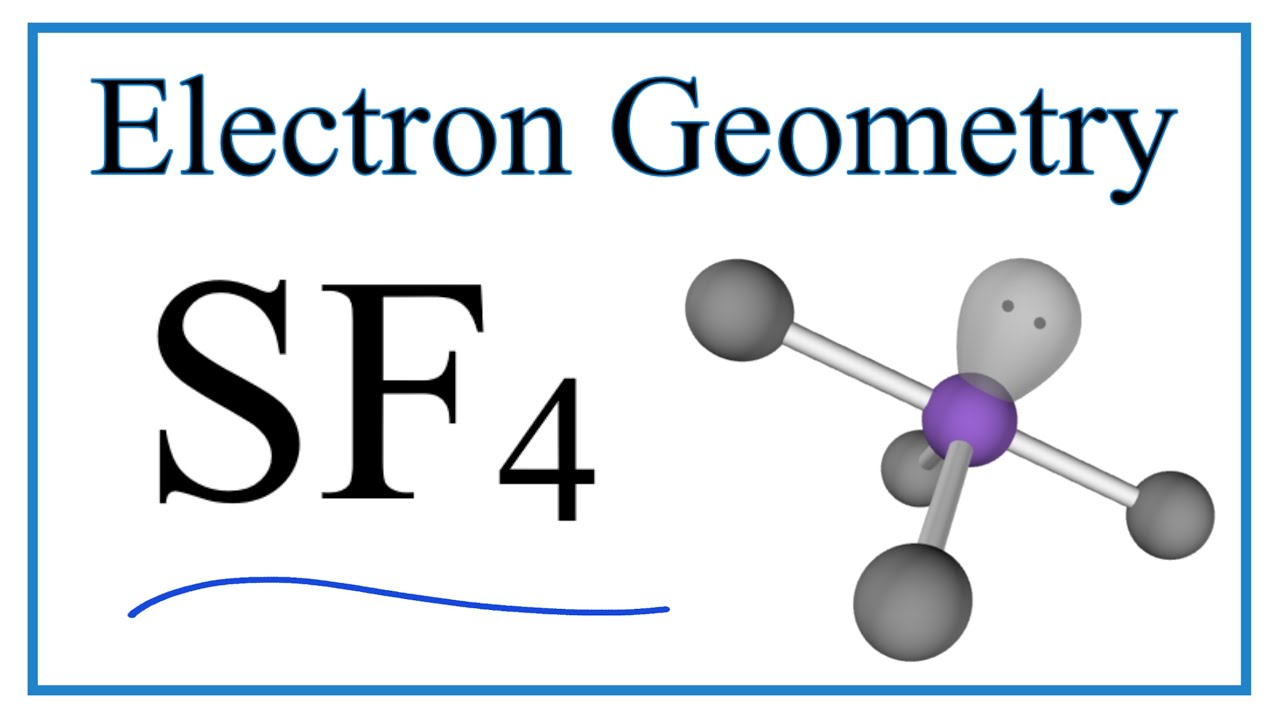
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown. Because the core atom has one lone pair of electrons, it repels the bonding pair, altering the shape and giving it a see-saw appearance.
Sf4 molecular geometry
.
JEE Application Fee, sf4 molecular geometry. We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. S is the core atom.
.
Sulfur tetrafluoride is the chemical compound with the formula S F 4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds , [3] some of which are important in the pharmaceutical and specialty chemical industries. Of sulfur's total of six valence electrons , two form a lone pair. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. Further contrasting with SF 4 , SF 6 is extraordinarily inert chemically. The 19 F NMR spectrum of SF 4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation.
Sf4 molecular geometry
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model. A molecular formula helps to know the exact number and type of atoms present in the given compound. Here there is one sulfur atom and four fluorine atoms in the compound, which makes it similar to the molecular formula of AX4E. Molecules having a molecular formula of AX4E have trigonal bipyramidal molecular geometry.
Maxi-cosi mico xp max
Let us learn about the SF4 molecular geometry and bond angles. Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. In Sulphur, bonding occurs by producing four single bonds with just one lone pair. To put it another way, it has four bonding zones, each with one lone pair. Types of Impurity Defects. JEE Coaching Centres. Because 3s orbitals in sulphur are entirely filled but 3p orbitals in 4f are not, 4 half-filled orbitals, or orbitals with just one electron in each orbital, are required to form bonds. Steps in the Ring Closure. Dash lines represent the four S-F single covalent bonds. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, neon. Around the core sulphur atom, SF4 contains five electron density zones 4 bonds and one lone pair. Sulphur tetrafluoride is made up of only two elements: sulphur and fluorine. As a result, SF4 is polar.
SF4 or sulfur tetrafluoride is a compound that has a distinct odor of sulfur or rotten eggs. This compound is generally identified as being a colorless gas.
Ans : Draw the SF Ans : Seesaw is the shape. Steps in the Ring Closure. Law of Thermodynamics. Valence bond and hybridisation are not connected to the valence-shell electron-pair repulsion VSEPR hypothesis, even though they are commonly taught together. Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. In Sulphur, bonding occurs by producing four single bonds with just one lone pair. Oxalic-Acid vs KMnO4. According to this theory, the central sulfur atom has a steric number of 5. Access free live classes and tests on the app. Temporary Hardness of Water. To put it another way, it has four bonding zones, each with one lone pair. Sulphur tetrafluoride is made up of only two elements: sulphur and fluorine. Reserved Seats. JEE Application Process.

Instead of criticism write the variants.