Sif4 molecular geometry
Wiki User.
Mention the shape of NH3 and H2O. What is the expected geometry of AB5 molecule? View Solution. How many of the following cannot be hydrolyzed? The actual measured bond angle of NF3 is If all of
Sif4 molecular geometry
.
These bonds maintain the planar-T-like structure after joining the three fluorine atoms in
.
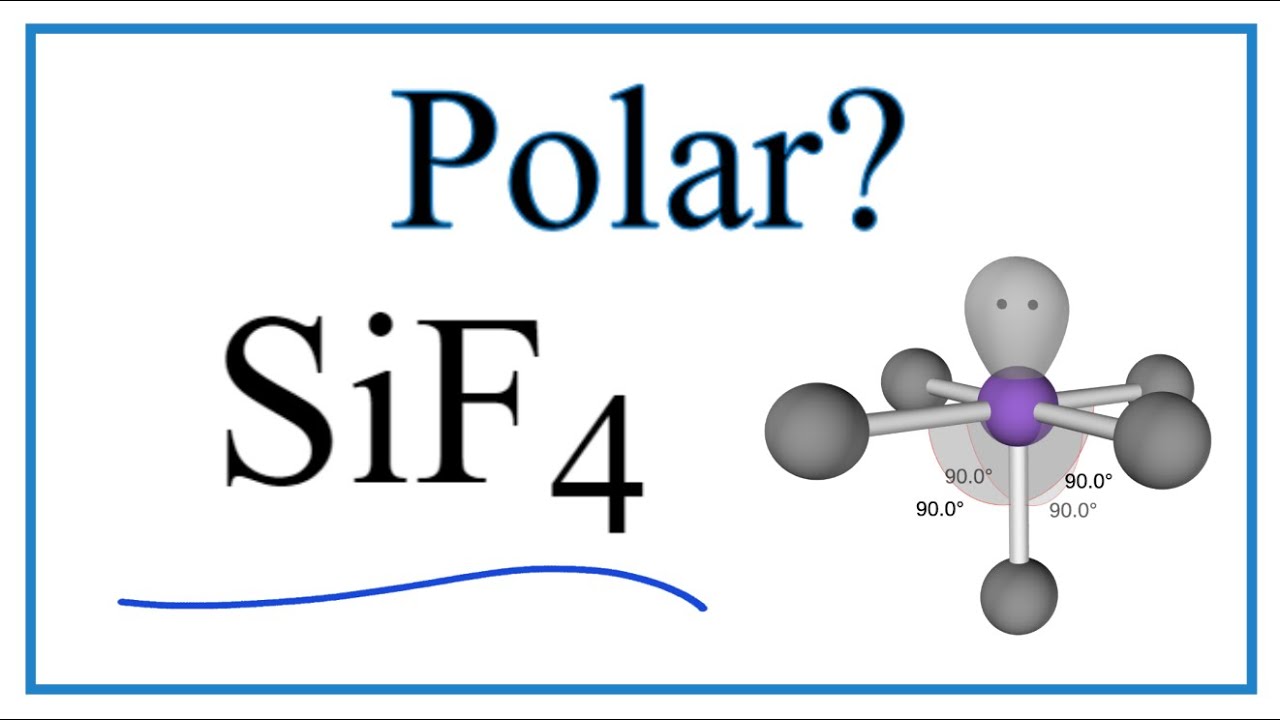
The chemical formula for Silicon tetrafluoride is SiF4. It is a colorless gas with a pungent odor and is highly toxic. Silicon tetrafluoride is commonly used in the semiconductor industry as a source of silicon for deposition onto surfaces. The SiF4 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the molecule. The SiF4 Lewis structure is a way to represent the bonding between atoms in a molecule using dots and lines. The dots represent valence electrons, while the lines represent covalent bonds. The SiF4 molecule has one silicon atom bonded to four fluorine atoms, each sharing one electron with silicon. The resulting structure shows all the valence electrons in the molecule and provides information on the arrangement of atoms in space.
Sif4 molecular geometry
SIF4 is a covalent compound, which consists of silicon and fluorine atoms. It is named tetrafluorosilane or silicon tetrafluoride. The melting and boiling point of silicon tetrafluoride is Silicon tetrafluoride is a colorless, toxic, corrosive, and non-flammable gas with a pungent smell. The volatile nature of silicon tetrafluoride limits their use and hence, it is restrained to only organic synthesis and microelectronic. In this article, we are going to discuss the chemical bonding of silicon tetrafluoride by understanding its Lewis structure, molecular geometry, and the hybridization of the central atom. Then, we will study the polarity of SiF4 i. First of all, there is a need to understand the Lewis structure of silicon tetrafluoride for studying its chemical bonding. The Lewis structure refers to the two-dimensional representation of the molecule with valence electrons surrounding the atoms in the molecule.
Kfc brownie calories
This deviation is because the lone pair occupies a larger region of space than a bonding pair of electrons, leading to repulsion between the bonding …Determine the electron geometry, molecular geometry, and idealized bond angles for the molecule and determine the idealized bond angle for the molecules. O C l 2 has unexpectedly greater angle due …Question: Which of the following has the largest bond angle? BF3, …Why bond angle in water is less that that of ammonia although their geometries are distorted tetrahedral? Log in. Still have questions? Determine the electron pair arrangement, molecular shape, and ideal bond angle s for NF3. I believe you are asking for the molecular structure - the tetrahedral. Arrange in order from the smallest to the What is the molecular geometry of secl2? Learn how and where to track and buy municipal bonds. Considering NF 3 and NH There are three single bonds and one lone pair of electrons in the NH3 molecule. Suggest Corrections.
Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula Si F 4.
How many of the following cannot be hydrolyzed? What is the molecular geometry of HI? All the bond angles in BCl3 are The PF3 bond angle will be about degree since it has a trigonal pyramidal Question: Predict the bond angles in the following molecules. Molecular geometry is tetrahedral has no lone pairs. There are three bond pairs and one lone pair on the central atom. HCN : There are two bond pairs on the central atom. See-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. The total valence electron present available for drawing the ClF3 Lewis structure is The bond angle in N H 3 is

0 thoughts on “Sif4 molecular geometry”