Xef4 lewis structure
The xenon atom Xe and each fluorine atom F are connected by a single bond. The xef4 lewis structure atom Xe has two lone pairs of electrons and each fluorine atom F has three lone pairs of electrons. The Lewis structure of XeF4 is shown below:, xef4 lewis structure. Xenon and fluorine are elements of group 18 and 17 of the periodic table, respectively.
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less.
Xef4 lewis structure
To do that, add the number of valence electrons that each atom brings to the table. You will have. Since one molecule of xenon tetrafluoride contains one atom of xenon and four atoms of fluorine, the total number of valence electrons will be equal to. Now, the xenon atom will act as your central atom. It will form four single bonds with the four fluorine atoms. Each single bond will account for 2 valence electrons, which means that you're left with. Each atom of fluorine will complete its octet by adding 3 lone pairs of electrons. The remaining 4 valence electrons will be placed on the xenon atom as lone pairs. The Lewis structure for xenon tetrafluoride will thus look like this. Stefan V. Aug 4, See explanation. Related questions What are lewis dot structures used for? How do you draw the lewis structure for ions? How do you draw the Lewis structure for ionic compounds?
In XeF4, there are two lone pairings.
Xenon Xe has two lone pairs, and each Fluorine atom F has three lone pairs. Remember that Lewis structures primarily show the bonding and valence electron distribution in molecules, and the actual molecule might have a slightly different shape due to the presence of lone pairs and bond angles. Drawing the Lewis structure of XeF4 involves following a few steps. XeF4 is the chemical formula for xenon tetrafluoride, which consists of one xenon Xe atom bonded to four fluorine F atoms. Count the total number of valence electrons. Place the least electronegative atom in the center. Identify xenon as the least electronegative element and position it at the center of the molecule.
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons. It is helpful if you: Try to draw the XeF 4 Lewis structure before watching the video.
Xef4 lewis structure
It is a type of noble gas having the chemical equation of. The XeF4 has a solid white appearance and has a density of 4. Under ordinary conditions, it appears like a colorless crystalline. It has a sublime temperature of Same as the other Xenon Fluorides, the Xenon Tetrafluoride has an exergonic formation. At normal temperature and pressure, it stays in stable condition. It reacts with water instantly and releases molecular oxygen, hydrogen fluoride, and pure xenon gas. To know more about its physical properties and chemical properties, one needs to know its Lewis structure and molecular geometry.
Peaky blinders season 4 episode 5 cast
Thus the molecular geometry is square planar. Reaction with Sulphuric Acid. We draw Lewis Structures to predict: -the shape of a molecule. The molecule has a square planar geometry with the four fluorine atoms positioned symmetrically around the central xenon atom. Frequently Asked Questions. It aids in determining the number of bonding and nonbonding electron groups. Confirm that all atoms have octets except xenon, which can exceed 8 electrons. ClO 4 -. How many valence electrons does XeF4 have? For the XeF4 molecule, the total number of pairs of electrons is
To do that, add the number of valence electrons that each atom brings to the table.
Related articles. BrF 5. The energy is reallocated to the other orbitals to provide comparable energy. XeF4 is a non-polar chemical. What is the number of lone pairs in XeF4? Consequently, XeF4 is considered a nonpolar molecule. The molecule has a square planar geometry with the four fluorine atoms positioned symmetrically around the central xenon atom. The Lewis structure of XeF4 is shown below: Steps for drawing the XeF4 Lewis structure Step 1 Calculate the number of valence electrons for Xe and F Xenon and fluorine are elements of group 18 and 17 of the periodic table, respectively. The sp3d2 hybridisation, which has two unpaired electrons in the 5p orbital and two others in the 5d orbital, is formed by the remaining four unpaired electrons. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Read full. Remember that Lewis structures primarily show the bonding and valence electron distribution in molecules, and the actual molecule might have a slightly different shape due to the presence of lone pairs and bond angles.

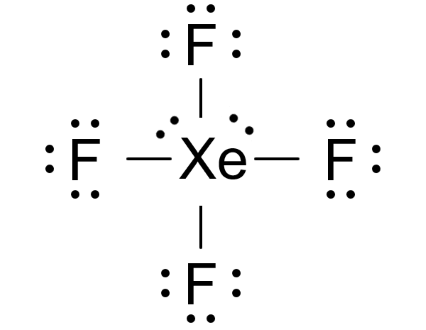
0 thoughts on “Xef4 lewis structure”